(2: 三峡水库生态系统湖北省野外科学观测研究站, 宜昌 443002)
(3: 云南师范大学高原地理过程与环境变化云南省重点实验室, 昆明 650500)
(2: Hubei Field Observation and Scientific Research Stations for Water Ecosystem in Three Gorges Reservoir, Yichang 443002, P.R. China)
(3: Yunnan Key Laboratory of Plateau Geographical Processes and Environmental Changes, Faculty of Geography, Yunnan Normal University, Kunming 650500, P.R. China)
湖泊、水库等淡水水体是甲烷(CH4)的重要排放源,其CH4最终通过冒泡排放、扩散排放、储存通量短时间释放和植物介导排放等方式排入大气[1]。全球内陆水体的CH4年排放量为398.1 Tg/a,其中来自湖泊和水库的CH4排放量分别贡献约37.9%和6.1%[2]。但目前估算的全球湖、库CH4排放仍需要进一步明晰其排放水平、变化性[3]。以往大量研究主要集中在CH4的扩散和冒泡(研究相对更晚、更少)2种排放方式[4]。CH4冒泡排放相当于直接传输厌氧沉积物中产生和储存的CH4至大气,在水柱中传输时因气泡的阻隔几乎没有物理或者化学消耗、或者与生物的相互作用,具有更高的CH4传输效率,是浅水水体中CH4排放的最重要途径[5-6],如亚热带湖泊太湖的冒泡通量占比在31%~71%[7-8],热喀斯特湖泊95%的CH4是通过冒泡排放[9];全球水库CH4总排放量中冒泡排放占比高达50%~90%[10-11]。但是,当前对湖、库水体CH4冒泡排放水平及其占比的估算依然存在很多不确定性。首先,湖、库沉积物中气体冒泡释放的气体组分及其比例具有时空差异性,冒泡气体组分有CH4、N2、O2、CO2等[12],而CH4气体含量的比例变化范围非常大,如湖泊中冒泡排放的气泡中CH4比例在1%~93%范围内变化;其次,CH4冒泡排放现象在时间和空间上均具有很大的随机性和异质性,而基于间歇性地和少量定点的观测无法体现整个水域的冒泡排放特征及水平,造成水体CH4冒泡通量和最终排放量估算的不确定性[13-14];再次,当前观测技术无法同时满足时间和空间上的分辨率要求,导致无法有效捕捉到水体CH4冒泡排放的时空变化特性,阻碍对不同时空尺度下CH4冒泡排放控制过程及影响因素的认识;对湖、库水体CH4冒泡排放的估算只能基于有限时段或者有限点位的平均值进行尺度扩展,且其准确性也不得而知,因而在估算区域或者全球等不同尺度上湖、库水体CH4排放时存在极大的不确定性[15]。
不同的水体类型以及其在不同的季节时段上,其CH4冒泡排放占水面总排放量的比例存在着巨大差异[16],以往多数关于湖、库水体CH4排放的研究中并没有区分扩散和冒泡通量,甚至忽略了冒泡排放,其中只有约52%的研究观测了CH4冒泡排放[13, 17],而能够在空间和时间分辨率上满足准确估算冒泡通量的观测则十分有限[16];观测设备的低时间空间分辨率则会导致对CH4冒泡排放的低估[15-16]。另外,有些湖泊湖滨带水生植物介导CH4排放和冒泡排放水平相当[18],对水生植物介导CH4排放的忽视则可能高估冒泡排放的贡献;仅估算扩散通量而忽视冒泡、植物介导的CH4排放等会导致实际总通量被大大低估[1]。近年来,随着对CH4冒泡通量重要性的认识,在湖、库等水体表面CH4通量的研究中采取了诸多方法来观测湖、库水体的CH4冒泡排放[17, 19]。当前虽然在一定程度上明确了湖、库水体中CH4冒泡通量的排放水平、时空变化规律及影响因素,但整体上对湖、库水体中CH4冒泡排放的过程和相互影响机制仍缺乏比较明确的认识[20]。
因此,本文系统回顾了已有湖、库水体中CH4冒泡的相关研究,对比了CH4冒泡排放观测技术,分析总结了湖、库水体CH4冒泡了的时空特征及其变异性,归纳了CH4冒泡排放的过程、影响因素及相关的模型,以期对未来湖、库水体CH4冒泡研究提出建议。
1 水体CH4冒泡排放的观测方法与技术用于水体CH4冒泡通量的传统计算方法主要包括基于浓度累计变化的浮箱法、生态系统质量守恒法和微气象法等[3],传统的观测手段则主要包括浮箱法(floating chamber)、倒置漏斗法(inverted funnel)等。但因为水体CH4冒泡排放在时间上具有零星随机性和空间分布上具有不规则性,存在一些显著的释放“热点”和“热期”,浮箱、倒置漏斗等手段则都只能提供定点或者线性区域的观测;因此,对于一个既定的研究对象如湖泊、水库或者其他水体类型,要想获得整个水域平面准确的、具有代表性的估算结果,需要布置相当数量的观测点位,同时设备还需要具备持续观测能力才能捕捉到CH4冒泡的时空变异性。其次,研究表明浮箱法有可能会改变近水面处的水力特性或者抑制箱内的水表微波从而造成结果偏差[21];而倒置漏斗法只能通过间歇性收集气泡并测定其中的CH4浓度,无法获取冒泡时间过程,而气泡中N2、CH4、O2等气体组分的比例会因为水体环境、运移时与周围水体的气体交换以及潜在的CH4氧化等影响而具有时空变异性。当然,近来诸多研究中结合了声纳探测技术(hydroacoustic detector)[22-23]、涡度相关技术(eddy covariance)[24]、合成孔径雷达遥感观测[25-27]或者不同技术相结合(如薄层边界法结合涡度相关技术)[7]等多种方法和技术等来估算较大区域内湖、库等水体中的CH4冒泡排放。不同方法均各有利弊,如表 1所示,如涡度相关技术在观测的时间尺度和空间覆盖上具有明显优势,可通过数据处理如小波分析法等区分量化CH4冒泡通量和扩散通量[24],但其设备昂贵且对应用条件的要求较为苛刻,并不能广泛地使用;声纳探测技术和遥感观测能够提供区域上较高分辨率的空间数据,结合定点实测数据校准,可以用来估算大面积水域的CH4冒泡通量[28]。
| 表 1 CH4冒泡通量观测技术方法及其优缺点 Tab. 1 The Pros and Cons of methods for monitoring CH4 ebullition |
总体上,以往研究更多地采用了经济且制作相对简便的倒置漏斗法、气泡收集装置和浮箱法,无法满足我们认识CH4冒泡过程和准确估算通量的需求。随着对CH4冒泡通量重要性的认识,各种基于传统方法的冒泡观测装置被开发并应用于湖、库等水体的CH4冒泡观测(表 1),促进了对水体CH4冒泡尺寸、排放过程和其影响机制的认识,提升了冒泡通量的估算准确性;如Varadharajan等[29]基于倒置漏斗和气体分压传感器组装的自动气泡收集装置,进行高时间精度和长时间序列观测并计算冒泡通量;Delwiche等[30-31]结合倒置漏斗和光学传感器,长期原位收集和监测CH4气泡尺寸并记录气泡上升的速度和时间,进而估算气泡的体积和CH4冒泡通量;Maher等[32]结合传统的漏斗收集装置、气压传感器和热敏CH4传感器,能够自动观测CH4冒泡速率和浓度;Thanh Duc等[33]基于浮箱法、倒置漏斗法以及气体浓度传感器等,实现水-气界面CH4的浓度变化自动在线观测并识别冒泡通量。
截止目前,水体CH4冒泡排放的观测方法及设备发展快速,但不同设备所得结果难以进行有效地相互验证,即使在同一观测点采用不同观测手段所得的结果也存在很大差异,导致根据不同时空尺度上观测所估算的通量结果甚至存在几个数量级的差异。因此,未来观测中还需要验证不同观测手段的观测结果的可靠性和代表性,研究所采用的观测流程、工具等至少需要可供参考的标准,才能进行有效的估算和评价单个或者区域尺度湖、库水体CH4冒泡排放的水平。
2 湖、库水体CH4冒泡排放的时空特征对湖、库水体CH4冒泡排放时空特征的认识有助于进一步明确其变化过程及驱动因素,也有助于准确估算和预测全球湖、库水体CH4排放水平。湖、库水体CH4冒泡排放在特定时间和空间上存在排放峰值,其时间变化与气温、水底温度、沉积物温度、低静水压力、低大气压等呈正相关关系;在空间上则主要发生在湖、库水体中水位较浅、具有高有机物含量、高有机物反应活性、高沉积速率的区域[35],CH4冒泡排放的空间变化会随着时间发生变化[36-37]。当前,只有少数针对单独湖泊或者水库水体CH4冒泡排放时空变化的对比分析[17, 35],缺乏对其特征在不同时空尺度上系统梳理和总结。下文将分别从不同时间(如小时、日、季节、年际等)和空间(如单个水体内部、不同水体间、不同气候带等)尺度分析总结湖、库水体CH4冒泡的特征。
2.1 CH4冒泡排放的时间变化特征湖、库水体CH4冒泡排放在时间上具有很大的偶发性,目前还无法定义一个通用时间尺度来描述CH4冒泡排放的频率、持续时间、强度等,也无法用类似描述扩散通量特征的方法表征冒泡所具有的时间变化特征并估算CH4冒泡通量[38]。冒泡本身也存在类型差异,如气泡尺寸、CH4产生途径、排放时空特征等,导致冒泡中CH4气体的含量及最终的CH4冒泡通量存在巨大差异;即使在同一点位上,不同时间点所测气泡中的CH4浓度差别也具有非常大的差异性,如存在着在短时间内的集中超量释放[39]。但多数研究主要基于单个冒泡事件(如根据单次冒泡的时间以及单次冒泡排放的气体总量等)来量化观测期间全部的冒泡事件及其持续时间以及其对水体CH4排放的贡献。可以借鉴的是,按照Walter等[9, 40]对西伯利亚地区的热喀斯特湖泊(thermokarst lake) CH4冒泡排放的研究,可将冒泡按特征分为背景冒泡(background bubbling,是指被随机布设的气泡收集装置所捕获的气泡释放的通量,其主要产生自表层沉积物,体现大部分湖面的低冒泡通量特征)、点源冒泡(point source bubbling,其气泡具有更高的CH4浓度,产生自较深层沉积物并通过沉积物表面上单个的细小的孔洞排放)和热点冒泡(hot spots bubbling,其气泡中所含CH4浓度高(>90%),具有非常高的冒泡速率且在冬季结冰的湖面上维持冒泡的孔洞)3种类型,各自观测和估算的时间尺度也是不同的,其中通过背景冒泡排放占全年排放CH4的24%,其CH4主要来自乙酸型途径,而后两者中CH4主要是来自CO2还原途径[40]。
通常,冒泡排放发生在分钟尺度上,但其持续时间可能在小时尺度、甚至日尺度[38, 41]。冒泡排放的时间尺度会随着水体环境因素的不同而变化,日尺度上水库CH4冒泡与库水位、大气压和温度显著相关[15, 42],在部分水库中具有显著的昼夜差异,如夜间CH4冒泡通量是白天的1.78倍[43];也有水库中CH4冒泡排放并无持续的昼夜变化规律[15]。湖泊CH4冒泡通量在小时及日尺度上随着温度(气温、表面水温和底泥温度)升高和气压降低分别呈指数增加和线性增加趋势[8];同时,湖泊CH4通量(包括扩散和冒泡)受日尺度上风速、光合有效辐射的影响,存在显著的昼夜变化趋势且昼、夜间的最大通量可能相差几倍[44];Gatun Lake湖泊CH4冒泡排放受风速的影响,排放峰值在当地时间的08:00-14:00[45]等。观测是否捕捉到冒泡及其排放峰值,会显著地影响到最终CH4排放量的估算结果;此外,观测设备的分辨率也是影响CH4冒泡排放通量估算的重要因素之一,不同时间尺度上观测估算的冒泡通量存在很大的差异,如水库CH4冒泡通量在分钟尺度(0~33×103 mg/(m2·d))和日尺度(0~5 mg/(m2·d))间相差2~3个数量级[42];不同空间分辨率的观测也会导致估算的CH4冒泡通量之间有数倍之差[35]。因此,需要进一步加强CH4冒泡通量观测体系构建、设备研发及观测数据尺度拓展关系建立等。
在季节尺度上,湖、库水体CH4冒泡排放的特征受地理位置、水体温度季节性变化导致的CH4产生水平、以及湖、库水位季节性变化及人工调节等因素的影响[46]。水位下降时期是水库CH4冒泡排放的“热期”,且其排放量可占CH4全年排放总量的90%以上[47-48],可能是因为水位对沉积物中气体储存的潜在影响,使得CH4冒泡排放的季节及年际变化性与其显著相关[49]。温度的季节性差异使得温暖季节的CH4冒泡排放要远高于寒冷季节[8, 50],但不同水体中CH4冒泡排放与温度的响应机制有所不同,如温带浅水湖泊中冒泡排放主要受沉积物温度季节性变化的影响以及低水位或者干枯持续时间等的影响[51];温带水库CH4冒泡通量的季节变化则主要受温度对CH4产生速率的影响而表现为秋、冬季低而春、夏季高的趋势,如德国Saar河蓄水段夏季CH4冒泡峰值是冬季的4~10倍[50];三峡支流库湾水域的CH4冒泡排放存在显著季节性差异且其排放峰值出现在6月份[39],主要与水库水位的下降有关[52];位于寒带、亚北极、青藏高原等高纬度、高海拔地区的湖泊因秋、冬季结冰而在冰盖下积累了大量的CH4,导致春、夏季冰盖融化后大量CH4集中冒泡排放[53];另外,因其对全球气候变化更敏感,变暖导致这些湖泊无冰期变长且大量的有机沉积物解冻,因而也将极大地改变湖泊CH4的产生和排放过程[54]。
在年际尺度上,湖泊CH4冒泡可能与湖泊生产力和气候变化间的相互作用有关[55],也与湖泊所接受的太阳能量输入强烈相关[56];水库中有机碳埋藏的年际水平能够反映其CH4冒泡的潜力[57];而库龄作为水库的重要特征,其对水库CH4排放的影响仍无定论[58-59];总体上因长期以来对CH4冒泡排放的忽略[17],目前缺乏有关湖、库CH4冒泡排放年际变化的研究;因此,未来需要通过长时间尺度且高频的观测来捕捉湖、库水体CH4冒泡的时空变化性[36]。
2.2 CH4冒泡排放的空间特征一般而言,在单个湖泊或者水库等较小空间尺度上,受沉积物垂向产甲烷能力和底物基质有机物含量等非均质性、水深、水位、水体理化性质的空间分布差异性等的影响,湖、库水体中CH4冒泡排放存在着相应的“热点”区域[60-61],且不同水域间也具有显著的空间变化[42, 51],如北极冻融湖泊、热带湖泊和水库等水体的CH4冒泡排放有着显著的排放“热点”[50];在浅水湖泊中,CH4冒泡排放通量最大值在湖泊中心部位,而湖泊内和湖泊间的空间差异性主要与沉积物碳的含量和类型有关,也与湖泊水深和观测点位(如湖滨带或湖心区)等有关[51]。而在深水环境中,上层水体的静水压力过大,导致沉积物中冒泡难以形成,相对浅水区因上层水体静水压力小、通常具有水生植物提供更多新鲜的内源有机碳,反而是CH4冒泡排放的主要区域[6];但不同研究中CH4冒泡排放区域与水深并无规律,如Rosa等[62]发现Samuel水库中CH4以冒泡排放为主的是水深小于5 m的水域;但也有研究发现小于50或100 m的水域以CH4冒泡排放为主[34, 63-64];另外有研究表明水库中靠近大坝的区域是CH4冒泡的“热点”,而冒泡通量则主要与泥沙沉积速率有关[65]。
在区域或者全球空间尺度上,湖、库水体CH4冒泡排放的差异既受其所在区域的自然地理和相应气候特征的影响,同时也与水体本身的特征如输入有机碳的类型和数量、库龄、水体生产力和入库流量的季节性变化、水位周期性波动及水体的混合动态等多种因素相关[17, 66]。目前,对温带、热带湖、库水体中CH4冒泡通量及其规律的研究相对比较多[67],也有较多对北方寒带、北极地区热喀斯特湖泊CH4冒泡通量的研究[9, 40, 68-69]。本研究统计的已有研究中湖、库空间分布如图 1及附图 Ⅰ所示,即使考虑到本文可能遗漏个别研究,其也能反映出当前对湖、库CH4冒泡排放研究十分有限且多数主要集中在北美和欧洲的现状;相较之下,相关研究在我国亟需开展。Miller等[67]研究表明,不同气候带水库CH4冒泡通量表现为温带水库>热带水库>寒带水库;而根据本研究统计分析(如表 2所示,基础信息见附表 Ⅰ),水库CH4冒泡通量及其占比在不同气候带间并没有显著性差异,湖泊也类似;不区分湖泊、水库的情形下(即不分类),只有CH4冒泡占比在寒带和热带间具有显著性差异(P < 0.01);同一气候带中,也仅有温带湖泊和水库的CH4冒泡通量具有显著差异(P < 0.01)。基于相关性分析,各气候带湖、库水体CH4冒泡通量及其占比的影响因素(水面面积、水深、纬度)各异;其中,温带湖泊和热带水库的冒泡通量受上述因素的影响均不显著;温带水库和寒带湖泊的冒泡通量分别与纬度呈正相关(R=0.48,P < 0.05)和线性负相关关系(R=-0.65,P < 0.01);而亚热带水库冒泡通量与其水体面积(R=-0.67,P < 0.01)显著相关,受水深(R=-0.47,P>0.05)和水库纬度分布(R=0.48,P>0.05)的影响均不显著;整体上,湖泊和水库CH4冒泡通量分别与纬度(R=-0.26,P < 0.05)和水深(R=-0.28,P < 0.05)呈负相关关系;湖、库水体中CH4冒泡排放占比的空间分布(附图 Ⅰ)则与水深(R=-0.27,P < 0.01)和水体所在纬度(R=0.25,P < 0.01))具有一定的线性关系。
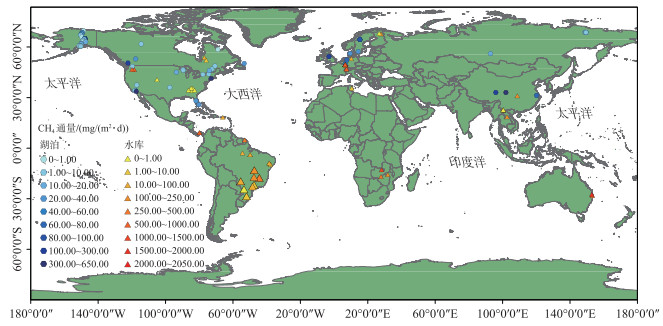
|
图 1 湖、库水体CH4冒泡通量的空间分布 (图中:○和△分别代表湖泊和水库水体,数据来自于文献,具体见附表 Ⅰ) Fig.1 CH4 ebullitive flux at the air-water interface from global lakes and reservoirs (The circles and triangles mark lakes and reservoirs, respectively; Related data source from references and details can be found in the attached Tab.Ⅰ) |
| 表 2 不同气候带湖、库水体CH4冒泡通量及其在CH4排放通量的占比 Tab. 2 CH4 ebullitive fluxes at the air-water interface from lakes and reservoirs across climatic zones and its ratio to the total CH4 emission |
综上,在大尺度上,湖、库水体CH4冒泡通量及冒泡占比并不受其所在气候带的影响,主要受湖、库水体所在地(纬度)的大气压变化、温度、风速和自身特性(如沉积物特性、CH4产生、水深、水面面积、水体分层)等因素的综合影响。由于有限的小尺度观测结果尺度扩展后得到大尺度上的结果仍有待进一步验证[70],短期、小范围的点位观测并不能反映单个湖泊或者水库整体冒泡通量的时空变化性[41, 51],低时空分辨率的观测可能错过冒泡排放事件,进而影响对湖、库CH4冒泡通量的准确估算和对CH4冒泡过程机制的认识[38]。因此,今后对于湖、库水体CH4冒泡通量的观测和研究,需要结合研究对象自身的特性、不同时空尺度上的主导因素,选择具有恰当时空分辨率的观测手段或构建合理的尺度扩展关系,进行长期连续的观测,才能进一步掌握湖、库水体CH4冒泡通量的时空变化并识别其在不同尺度上的驱动机制[41, 71]。
3 CH4气泡的生长、排放过程及模型应用 3.1 沉积物中CH4气泡的形成-生长过程及影响因素理论上,沉积物中CH4气泡的形成需要具备3个条件:首先,沉积物具有较高的CH4产生潜力及速率,气泡中CH4含量则主要取决于沉积物的产甲烷过程,主要受有机质质量、温度、厌氧环境等因素限制[72];其次,经过氧化消耗后,要有足够的CH4净产生量进入孔隙水中[73];第三,孔隙水中气泡的形成需要考虑不同溶解气体(如N2、O2、CO2和CH4等)总气压,由于CH4具有微溶于水的特性,只有当大量气体(如CH4等)进入沉积物孔隙水,其中的总气压由于过饱和大于或等于沉积物所在位置的环境压力(静水压力+大气压)时,才会通过成核(Nucleation)作用在沉积物孔隙水中形成微气泡[74-75];形成的微气泡与周围孔隙水溶解CH4浓度维持动态平衡,该过程可用亨利定律(Herry's Law)来描述并用过饱和比率(supersaturation ratio, ζ)来衡量(ζ>0,气泡产生;ζ=0,气-液相平衡)[76]。
气泡生长始于微气泡接受来自周围孔隙水中大量过饱和CH4气体,体积变大的气泡进一步弹性挤压沉积物基质或使沉积物膨胀破裂[77];相反,沉积物中CH4低产生量、微气泡周围CH4低溶解浓度以及沉积物颗粒的强触点压力对冒泡内部膨胀力的抗衡均会导致气泡生长的停滞[78]。沉积物中气泡生长机制可细分为两种情形:(1)在孔隙尺度上,气泡形成后会迅速增长并达到孔隙尺寸,气泡生长速率由孔隙水CH4向气泡中的扩散速率决定,扩散速率则取决于孔隙水与气泡中CH4浓度差[78-79];(2)在大于孔隙尺度的空间上,沉积物基质中气泡的生长具有多种机制:①气泡占据孔隙空间而挤压出孔隙水,沉积物基质空间孔隙结构保持不变;②气泡生长导致沉积物基质弹/塑性变形进而将气泡排出孔隙;③气泡生长破坏沉积物固体颗粒间的凝聚力和粘合力并导致沉积物基质的破裂变形,气泡则占据固体颗粒原来的空间[77]。基于以上不同形成机制,沉积物中气泡总体上可以分为3类:Ⅰ类气泡(直径d < 0.1 mm),是微气泡通过侵入毛管孔隙进行生长,所形成的气泡则完全包含在沉积物固体颗粒间隙;相较而言,砂质沉积物比黏土沉积物更容易形成此类气泡;Ⅱ类气泡(0.1 mm<d < 1 mm)则是气泡尺寸大于孔隙尺寸的中型气泡,其挤出孔隙水而占据其空间,但沉积物仍旧保持原有结构;Ⅲ类气泡(d>1 mm)是在低剪切强度(< 100 Pa)的沉积物中由气体完全填充并替代部分固体颗粒空间条件下形成的[80];一般情况下,Ⅲ类气泡在细粒的泥质沉积物中最为常见;有机沉积物中冒泡排放的气泡也多为大尺寸气泡,气泡在沉积物孔隙中的生长、传输受沉积物颗粒结合形成的多孔网状结构的影响[64]。
在沉积物CH4净产生量满足的前提下,CH4气泡的生长过程及其尺寸分布、体积和形状等主要取决于沉积物的物理特性(如粒度、矿物组成、粘滞性、孔隙率等)[72, 81]、气泡表面有效压力(取决于孔隙水压力与包括大气压力、静水压力和沉积物重力等垂向压力的平衡[78])的影响。在粗颗粒沉积物中,毛细管是储气和气泡生长的主要空间,微气泡主要通过侵入毛细管孔挤出孔隙水而成长和迁移的[82],气泡在其中的生长变化过程可通过耦合沉积物孔隙空间的气-液两相微观离散元模型描述[83]。在细颗粒沉积物中,气泡侵入毛管时所需克服的压力要远大于克服沉积物颗粒间的粘结力,气泡(至少10倍于沉积物粒径)的形成和生长通过对周围沉积物的塑性/弹性挤压来获得空间,气泡更大时甚至会造成沉积物裂缝[84]。当前,针对气泡生长过程及影响因素已有很多相关研究,如沉积物颗粒间如静电、有机络合、水基吸引等作用力对气泡生长的影响可用沉积物线性弹性断裂机理(linear elastic fracture mechanics,LEFM)解释;沉积物中不同形状气泡生长时间及尺寸大小可用耦合LEFM的准稳态反应扩散模型(quasi-steady state reaction-diffusion model)确定[79];气泡在沉积物裂缝产生时的瞬时生长问题可以通过基于LEFM的有限元模型(finite-element model)分析,其考虑了孔隙水中溶解CH4的供应、气泡生长时间和尺寸、沉积物基于LEFM原理的弹性膨胀以及气泡成长使气泡顶端沉积物均匀断裂等过程[80, 85];Katsman等提出的耦合力学/反应-气泡传输模型,则能够给出细颗粒均质沉积物中气泡生长过程中的气泡形状、尺寸以及其时间变化过程,还可以通过对沉积物中气泡最终形态特征的模拟,量化从沉积物中产生并经气泡在水体传输最终释放到大气的CH4总量[86]。
3.2 气泡在沉积物-水体中的迁移、排放过程及影响因素在沉积物中的成核点产生、成长的气泡,既可能因尺寸被困在沉积物孔隙中,也会因浮力而迁移上升[87],而上升过程则由伪浮力(pseudo-buoyancy)控制下的沉积物粘弹性形变及断裂过程主导[78]。孔隙水压力和垂向总压力(静水压力、大气压等)平衡后的有效压力影响气泡的迁移过程[79],沉积物基质的机械特性如粒度、矿物组成、粘滞性等会影响到气泡尺寸、形状,进而影响到气泡的上升路径、速率和气泡内气体组分[77, 88-89]。其中,气泡在沉积物孔隙尺度上迁移和滞留时气泡尺寸变化及其对滞留气泡比例、剩余气体饱和度、沉积物孔隙水力导度的影响可以借助孔隙网格模型(pore-network model)分析[87]。单个气泡开始上升时的初始尺寸和上升速度可基于粘弹性断裂-气泡上升模型(viscoelastic-fracture bubble-rise model)利用沉积物的粘弹性来估算[88],但由于该模型没有考虑气体产生、气泡与周围沉积物间的物质传输等过程,因而并不适用于已经形成沉积物气泡通道和裂缝中的气泡上升过程[88]。
通常,浅水中低静水压力下气泡尺寸较大且上升速度较快,而高静水压力下气泡尺寸和上升速度均较小。在一定的深水条件下,沉积物中产生的CH4等碳氢化合物气泡进入水柱后气泡表面会很快形成水合物膜[90];水合物膜主要发生在CH4气体饱和的气泡-水界面,形成完整气泡水合物膜的气泡尺寸及时间等则受水深、温度、气泡内CH4气体分压及水体环境中的CH4浓度等因素的限制[5, 91]。气泡内的CH4可以通过气泡上的水合物膜裂隙与周围水体进行扩散交换,而水合物膜的形成则大大减弱气泡中CH4的快速溶解,从而使得深水中CH4气泡不致破裂而更多地向上传输、排放[92]。目前,单个气泡从沉积物进入水柱后的一系列特征如气泡尺寸、所含气体组分的物质传输系数、扩散特性和溶解特性、上升速率等的变化过程和主要影响因素都可以根据单气泡模型(single bubble model)进行模拟和分析[5]。CH4气泡水合物膜的形成和维持是水合物膜在气泡外部不断溶解扩散而在内部不断形成的动态过程,该过程降低了气泡内部的CH4气体分压,一定程度上抵消了气泡上升后静水压力降低对气泡的影响,有助于防止气泡在上升过程因体积增大过快导致的破裂而使其在水中存在更长时间并最终在水-气界面扩散释放[93],也影响气泡在水中的上升速度、物质传输等特性和最终的CH4冒泡通量[91]。除此之外,气泡上升引起的羽流带动富含溶解CH4的水体上升到表层,在一定程度上促进了水体CH4的扩散排放[92]。当前,为深化和明确对具有不均匀沉积物、多气泡产生等情形的湖、库水体中CH4气泡产生、迁移、排放的过程,可借助Darmana等[94]、Lau等[95]、Battistella等[96]先后改进的离散气泡模型(discrete bubble model),对均质或异质性介质中成核点位上气泡的产生-长大-脱离成核点后上升等气泡动力学过程、气泡羽流中多气泡间的相互作用(如气泡的碰撞、合并和碎裂)、气泡中物质传输(如物理传输、化学反应)等过程进行模拟研究[96]。
湖、库CH4冒泡通量受多种因素的影响,CH4气泡的产生取决于沉积物CH4生产率,温度和水体富营养化程度等则影响CH4生产率[55];CH4气泡一般主要来自表层10~20 cm沉积物[50, 97];水深与其呈强烈负相关关系,尤其是在浅水(< 2 m),当水深超过10 m后冒泡通量则很小[97-98];气泡尺寸则强烈影响着气泡的上升速度进而影响着气泡中CH4的溶解消耗[6],且气泡尺寸和上升速度的响应关系受水体污染物影响[5];不同水深条件下气泡内部相对稳定的气体留存比例受气泡尺寸的影响,并不会因气泡尺寸的增大而上升[64, 88];通常,来自少数大尺寸气泡的CH4贡献了绝大部分的冒泡通量,气泡尺寸分布比气泡数量能够更准确地估算CH4冒泡排放量[64];而气泡的迁移过程与由风速、水体内部压力梯度以及水下地形等因素决定的沉积物表面水流剪切力密切相关[97]。由此可见,对于湖、库水体CH4冒泡通量的估算,需要综合考虑CH4产生、气泡生长-迁移的过程及影响因素,而目前可使用的基于过程的模型包括bLake4Me、ALBM等;其中bLake4Me是基于多气体、多模块和多过程提出的湖泊生物地球化学模型,能在垂向上对沉积物-水柱中CH4的产生、氧化和传输进行模拟;其中考虑了气泡传输时气体组分(N2、O2、CO2和CH4)与周围水体的气体交换以及多因素(静水压力、气泡在水柱中的尺寸、水体中CH4气体溶解浓度)对气泡中气体浓度的影响[99];一维ALBM模型在bLake4Me基础上主要改进了热喀斯特湖泊中沉积物冻融过程、深层沉积物易分解有机碳的转移和矿化过程、热喀斯特活动引起的有机碳输入过程和光化学矿化影响溶解有机碳的降解过程,使得模型能够更广泛地应用到北方湖泊的物理生物地球化学过程的模拟[100-101]。相较之下,LAKE是首个垂向一维湖泊甲烷收支模型,其综合考虑了湖泊热力学、水力学、湍流闭合、沉积物水-热变化过程、CO2、CH4和O2等气体的生物地球化学循环以及扩散和冒泡传输机制,气泡模块耦合了McGinnis等开发的气泡模型,可实现沉积物-水柱中的CH4在垂向传输过程的模拟[102-103]。
3.3 基于过程的湖、库沉积物-水体CH4冒泡模型框架当前对水下沉积物中CH4气泡形成相关机理的研究已经比较深入,但对沉积物-水体中CH4气泡的迁移-排放过程机理及相关模型多来自于理想状态下或对海洋的研究,模型众多且多数只针对特定水体或者冒泡传输的部分过程。未来,在深入认识湖、库沉积物-水体CH4气泡形成-生长-迁移-释放全过程的基础上,需验证各模型在湖、库水体中的适用性并发展和完善,实现不同过程模型间的耦合和相互验证。通过将CH4冒泡过程模型嵌套在相关的湖、库沉积物-水体CH4动态模型、碳循环模型和物理-生物地球化学过程模型等当中,以深化对湖、库中物质-能量-动量循环过程及机理的认识,提升区域和全球尺度上湖、库水体CH4排放估算的准确性并预测其对气候变化、人类干扰活动的响应。
本研究根据湖、库沉积物CH4产生和氧化、沉积物-水-大气中CH4扩散排放、沉积物中CH4储存、气泡产生-成长、CH4气泡在沉积物-水体中的迁移等过程以及主要的影响因素,提出基于过程的湖、库沉积物-水体CH4冒泡模型框架,该框架包括沉积物-水体水热动力模块、沉积物CH4产生-氧化模块、孔隙水CH4扩散传输模块、水柱CH4扩散传输-氧化模块、沉积物CH4储存和气泡产生模块、沉积物CH4气泡传输模块以及水体CH4气泡传输共7个模块(图 2),各模块中关键过程的相关参数及其数学公式见附件3,以期为未来CH4冒泡研究和模型开发提供思路借鉴。
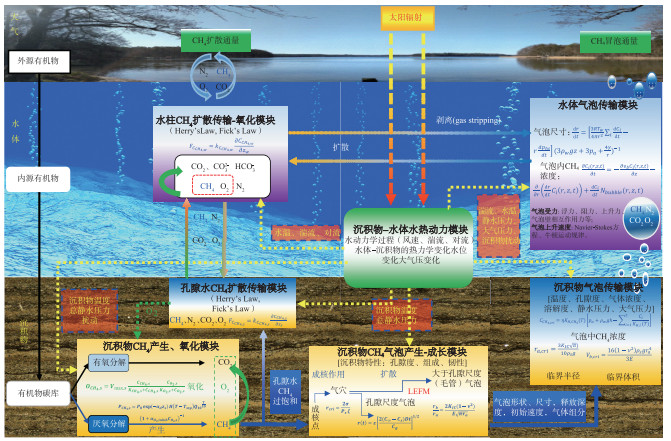
|
图 2 湖、库沉积物-水体CH4冒泡模型框架 Fig.2 The conceptional scheme of CH4 ebullition in the sediment-water continuum in lakes and reservoirs |
湖、库水生态系统CH4冒泡排放的精确观测、排放时空变化规律及其排放机制认识,对于准确估算区域、全球尺度CH4排放和应对全球变化等方面具有重要的意义。本文系统比较了常用CH4冒泡的观测方法优缺点,分析总结了全球湖、库水体CH4冒泡通量的时空分布规律,梳理了有关沉积物-水体CH4冒泡排放的机理、影响因素和模型,以期为未来湖、库水体CH4冒泡通量的观测研究提供思路和参考。基于此,本文认为,当前可用于CH4冒泡通量观测在方法、技术众多,但观测结果的代表性和相互之间的验证不足;已有对水体CH4冒泡排放机制和模型多源于对海洋的相关研究,缺乏对易受人类活动及气候变化影响的湖、库水体CH4冒泡排放过程机理及影响因素的全面认识,导致湖、库水体乃至全球CH4收支估算结果的巨大不确定性。因此,在未来湖、库水生态系统CH4排放的观测和研究中,CH4冒泡排放应与扩散排放具有同等的重要性,亟需在观测技术、估算方法等方面进行探索和研究。本文提出未来应从以下方面开展更多研究:
1) 基于CH4冒泡特征进行分类(如背景冒泡、点源冒泡和热点冒泡),分类研究湖、库水体CH4冒泡排放的时空尺度及特征、排放水平和气泡产生-传输-排放的机制及过程。
2) 验证不同观测技术和方法观测结果的适用性,解析其不确定性,设计、开发能够进行高频、长期和大范围观测水体CH4冒泡的设备。
3) 由于湖、库水体CH4冒泡排放本身极具时空异质性,因此建议按照相关要素如面积、水深等划分湖、库水体级别,根据不同观测方法的适用性及观测数据精度,构建适应性的各级湖、库CH4冒泡监测的标准体系和方案,扩展和验证不同尺度上的冒泡通量,以优化监测结果的代表性和可比对性。
4) 在系统的观测方法及体系基础上,加强对气候变化和人类活动综合影响下湖、库CH4冒泡排放的研究,如气温上升、水体富营养化、管理水平等因素耦合对典型湖、库CH4冒泡排放过程的影响等。
5) 验证已有模型在湖、库CH4冒泡研究中的适用性,结合更全面、精确的观测,发展基于湖、库沉积物-水体CH4气泡形成-生长-迁移-释放全过程的模型或模块,通过模型嵌套及融合, 深化对湖、库CH4动态过程及其对变化环境响应的研究。
5 附录附图 Ⅰ、附表 Ⅰ以及湖、库沉积物-水体CH4冒泡模型框架中各模块关键参数的数学公式见电子版(DOI: 10.18307/2024.0201)。
附件1
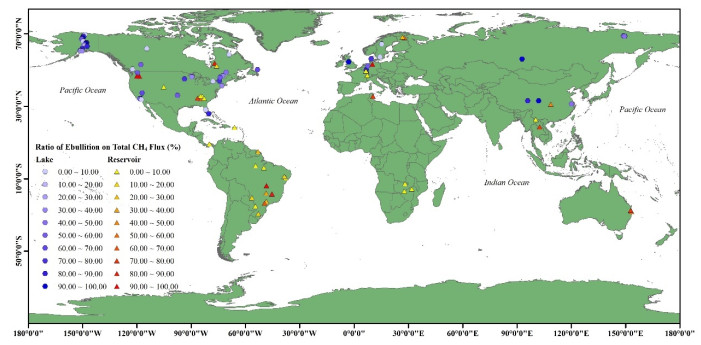
|
附图Ⅰ 湖、库水体CH4冒泡排放占比的空间分布 (图中:○和△分别代表湖泊和水库水体,数据来自于文献,具体见附表 Ⅰ) AttachedFig1 Global spatial distribution of ebullition/total CH4 flux from lakes and reservoirs. The circles and triangles represent lakes and reservoirs, respectively; related data source from references and details can be found in the attachment |
附件2
| 附表Ⅰ 不同气候带湖、库水体CH4冒泡通量* Appendix Ⅰ Methane ebullition fluxes from lakes and reservoirs across different climatic zones |
附件3
湖、库沉积物-水体CH4冒泡模型框架各模块中关键过程的相关参数及其数学公式
(1)沉积物-水体中水、热动力模块
①水体水动力过程:根据雷诺平均平流扩散方程,水体的水平动量通量受水体水平出入流引起的对流、湍流扩散、底部的垂直通量、由科里奥利力和水平压力梯度引起的通量变化和由水位变化引起的动量变化等因素的影响,其变化过程可表述为公式(1)[42]:
| $ \frac{{\partial \overline {{M_h}} }}{{\partial t}} = - \frac{1}{A}\mathop \smallint _{{{\rm{\Gamma }}_{A\left( \xi \right)}}}\left( {{u_h}n} \right)dl + \frac{1}{{A{h^2}}}\frac{\partial }{{\partial \xi }}\left( {A{k_f}\frac{{\partial \overline {{M_h}} }}{{\partial \xi }}} \right) + \frac{1}{{Ah}}\frac{{dA}}{{d\xi }}{F_{tz, b}}\left( \xi \right) + {R_f}\left( {{M_h}} \right) + \left[ {\frac{\xi }{h}\frac{{dh}}{{dt}} - \frac{{{B_s}}}{h}} \right]\frac{{\partial \overline {{M_h}} }}{{\partial \xi }} $ | (1) |
式中
②沉积物-水体热力学过程:水柱中的热量来源于太阳辐射,水体中热传导主要以风力驱动的涡流扩散、分子扩散和水体对流主导;沉积物中的热量主要来源于上覆水热量的分子扩散,水柱底层和沉积物表层的热传导是假定平衡的,而沉积物底层的热通量则假定为0。
水柱中的热力过程可表述为[43]:
| $ \frac{{\partial {T_w}}}{{\partial t}} = \frac{1}{A}\frac{\partial }{{\partial z}}\left( {A\left( {{D_m} + {D_e}} \right)\frac{{\partial {T_w}}}{{\partial z}}} \right) + \frac{1}{A}\frac{\partial }{{{\rho _w}{c_{pw}}}}\frac{{\partial \left[ {{\rm{\Phi }}A} \right]}}{{\partial z}} $ | (2) |
式中Tw水温,t为时间,z为水深,A为水体对应水深处水平面截面面积,Dm为水体的分子扩散系数;De为风力驱动的涡流扩散系数;
沉积物温度变化过程可表述为[44]:
| $ {c_{vs}}\frac{{\partial {T_s}}}{{\partial t}} = \frac{\partial }{{\partial z}}\left( {{k_s}\frac{{\partial {T_s}}}{{\partial z}}} \right) $ | (3) |
式中
(2)沉积物CH4产生-氧化模块[42]
①湖、库沉积物中CH4产生速率主要受不稳定有机碳含量、温度的影响,可表述为:
| $ {P_{C{H_4}, s}} = {P_0}{\rm{exp}}\left( { - {\alpha _d}{z_s}} \right)H\left( {T - {T_{mp}}} \right){Q_{10}}^{T/10}{\left( {1 + {\alpha _{{O_2}, inhib}}{C_{{O_2}, s}}} \right)^{ - 1}} $ | (4) |
式中
②湖、库沉积物中CH4氧化消耗主要发生在沉积物表层,表层中O2浓度随深度呈指数下降,CH4有氧氧化可根据Michaelis-Menten动力学模型估算:
| $ {O_{C{H_4}, s}} = {V_{max, s}}\frac{{{C_{C{H_4}, s}}}}{{{K_{C{H_4}, s}} + {C_{C{H_4}, s}}}}\frac{{{C_{{O_2}, s}}}}{{{K_{{O_2}, s}} + {C_{{O_2}, s}}}} $ | (5) |
式中
③沉积物中CH4气体浓度的变化与其产生速率、消耗速率、扩散传输、气泡传输等有关,不考虑其在沉积物横向传输的情况下,其可用公式(6)表述:
| $ \frac{{\partial {C_{C{H_{4, s}}}}}}{{\partial t}} = \frac{\partial }{{\partial z}}{k_{{C_{C{H_{4, s}}}}}}\frac{{\partial {C_{C{H_{4, s}}}}}}{{\partial {z_s}}} + {P_{{C_{C{H_{4, s}}}}}} - {E_{{C_{C{H_{4, s}}}}}} - {O_{{C_{C{H_{4, s}}}}}} $ | (6) |
式中
(3)孔隙水CH4扩散传输模块
孔隙水中CH4的扩散传输符合Fick定律,主要受沉积物不同深度处CH4浓度和其扩散系数的影响,其扩散通量
| $ {F_{{C_{C{H_{4, s}}}}}} = {k_{{C_{C{H_{4, s}}}}}}\frac{{\partial {C_{C{H_{4, s}}}}}}{{\partial {z_s}}} $ | (7) |
(4)水柱CH4扩散传输-氧化模块[42]
类似于孔隙水中CH4的扩散传输,Fick定律也适用于沉积物-水界面CH4扩散释放得到水柱底层CH4初始浓度及通量、CH4在水体中扩散传输速率、水-气界面的通量的估算;CH4水柱中扩散传输与扩散系数、不同水深处的浓度差、水-气界面处表层水体和大气中的CH4的浓度差等相关;水柱中CH4的氧化速率则利用Michaelis-Menten动力学模型来估算:
| $ {O_{C{H_4}, w}} = {V_{max, w}}\frac{{{C_{C{H_4}, w}}}}{{{K_{C{H_4}, w}} + {C_{C{H_4}, w}}}}\frac{{{C_{{O_2}, w}}}}{{{K_{{O_2}, w}} + {C_{{O_2}, w}}}} $ | (8) |
式中
(5)沉积物CH4气泡产生-成长模块
沉积物形成CH4气泡需要有成核点(如悬浮颗粒、沉积物缝隙或者自由的小气泡),即气穴,而气穴半径需要大于或者等于其所在沉积物条件下的阈值才能使气泡形成并生长,小于此阈值半径的气泡会因为溶解而迅速消失;其中,气穴半径阈值可用公式(9)表述[45]:
| $ {r_{critical}} = \frac{{2\sigma }}{{{P_{s}}\zeta }} $ | (9) |
式中
气泡在成核点形成以后气泡内跟周围孔隙水扩散交换而长大。在孔隙尺度上,气泡有效半径的变化过程可用公式(10)描述[46]:
| $ r\left( t \right) = \varepsilon {\left[ {\frac{{2\left( {{C_\infty } - {C_s}} \right)Dt}}{{{C_g}}}} \right]^{1/2}} $ | (10) |
式中
在大于孔隙度尺度时,气泡通过侵入毛细管中挤压毛管水生长;气泡进一步长大时,由于沉积物的类型不同,气泡生长的尺寸、形状等均有所差异。根据沉积物拉伸断裂线性弹性机理(LEFM),气泡在沉积物裂缝中的形状类似“圆盘”,且进一步生长在沉积物成扁球体,其长轴半径ra和短轴半径rb的关系符合公式(11)[46]:
| $ \frac{{{r_b}}}{{{r_a}}} = \frac{{4{P_f}\left( {1 - {\nu ^2}} \right)}}{{\pi E}} = \frac{{2{K_{IC}}\left( {1 - {\nu ^2}} \right)}}{{E\sqrt {\pi {r_a}} }} $ | (11) |
式中Pf为沉积物断裂所需的额外气体压力,ν为泊松比,E、KIC分别为沉积物的杨氏模量和拉伸断裂的韧度。
(6)沉积物CH4气泡传输模块
气泡成长达到一定的尺寸后开始在沉积物中运动上升,受沉积物密度、沉积物裂缝的强度、重力因素的影响;该过程中伴随着气泡体积、形状的变化,其中,气泡上升的临界半径
| $ {r_{a, critial}} = \frac{{3{K_{IC}}\sqrt \pi )}}{{10{\rho _s}g}} $ | (12) |
| $ {V_{b, critical}} = \frac{{16\left( {1 - {\nu ^2}} \right){\rho _s}gr_a^4}}{{3E}} $ | (13) |
式中
气泡中CH4气体的浓度
| $ C_{C H_{4, \text { critical }}}=\eta K_{H, C H_4}(T)\left[p_a+\rho_w g h-\sum_{i=1}^3 \frac{C_i}{K_{H, i}(T)}\right] $ | (14) |
式中η为沉积物孔隙度,T为沉积物温度,
(7)水体CH4气泡传输模块
当气泡突破沉积物-水界面后,会在水柱中上升并迅速到达水-气界面释放。其中,气泡上升过程中其尺寸、数量、气泡内的气体浓度会随着时间、在水柱中位置而发生变化。其中,气泡尺寸(r)和气泡数量
| $ \frac{{dr}}{{dt}} = \left[ {\frac{{3R{T_w}}}{{4\pi {r^2}}}\mathop \sum \limits_i \frac{{d{C_i}}}{{dt}} - r\frac{{d{p_{hs}}}}{{dt}}} \right]{\left( {3{\rho _w}gz + 3{p_a} + \frac{{4\gamma }}{r}} \right)^{ - 1}} $ | (15) |
| $ {N_{bubble}}\left( {r, z, t} \right) = \frac{{3R{T_w}}}{{4\pi {r^2}}}\mathop \sum \limits_i {C_i}\left( {r, z, t} \right) $ | (16) |
气泡在上升过程中,气泡内外气体分压差导致气泡与水柱中的气体发生扩散交换,因而单个气泡中CH4、N2、O2、CO2的浓度变化为[48]:
| $ \frac{{d{C_i}}}{{dt}} = - 4\pi r{D_i}N{u_i}\left[ {{S_i}m_i^b\left( {{\rho _w}gz + {p_a} + \frac{{2\gamma }}{r}} \right) - {C_{i, w}}} \right] $ | (17) |
最终,某时刻某一深度下特定半径所有气泡中CH4、N2、O2、CO2等气体的浓度可用二维连续方程计算来确定[49]:
| $ \frac{{\partial {C_i}\left( {r, z, t} \right)}}{{\partial t}} = - \frac{{\partial {\upsilon _b}{C_i}\left( {r, z, t} \right)}}{{\partial z}} - \frac{\partial }{{\partial r}}\left( {\frac{{dr}}{{dt}}{C_i}\left( {r, z, t} \right)} \right) + \frac{{d{C_i}}}{{dt}}{N_{bubble}}\left( {r, z, t} \right) $ | (18) |
公式(15)-(18)中,t为时间,z为气泡所在处的水深,R、g分别指气体常数和重力加速度,
附录参考文献
[1] Duchemin É, Lucotte M, Canuel R et al. First assessment of methane and carbon dioxide emissions from shallow and deep zones of boreal reservoirs upon ice break-up. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 2006, 11(1): 9-19. DOI: 10.1111/j.1440-1770.2006.00285.x.
[2] Teodoru CR, Bastien J, Bonneville MC et al. The net carbon footprint of a newly created boreal hydroelectric reservoir. Global Biogeochemical Cycles, 2012, 26(2). DOI: 10.1029/2011gb004187.
[3] DelSontro T, McGinnis DF, Sobek S et al. Extreme methane emissions from a Swiss hydropower reservoir: Contribution from bubbling sediments. Environmental Science & Technology, 2010, 44(7): 2419-2425. DOI: 10.1021/es9031369.
[4] Grinham A, Dunbabin M, Gale D et al. Quantification of ebullitive and diffusive methane release to atmosphere from a water storage. Atmospheric Environment, 2011, 45(39): 7166-7173. DOI: 10.1016/j.atmosenv.2011.09.011.
[5] Maeck A, DelSontro T, McGinnis D et al. Sediment trapping by dams creates methane emission hot spots. Environmental Science & Technology, 2013, 47(15): 8130-8137. DOI: 10.1021/es4003907.
[6] Maeck A, Hofmann H, Lorke A. Pumping methane out of aquatic sediments–ebullition forcing mechanisms in an impounded river. Biogeosciences, 2014, 11(11): 2925-2938. DOI: 10.5194/bg-11-2925-2014.
[7] DelSontro T, McGinnis DF, Wehrli B et al. Size does matter: Importance of large bubbles and small-scale hot spots for methane transport. Environmental Science & Technology, 2015, 49(3): 1268-1276. DOI: 10.1021/es5054286.
[8] Wilkinson J, Maeck A, Alshboul Z et al. Continuous seasonal river Ebullition measurements linked to sediment methane formation. Environmental Science & Technology, 2015, 49(22): 13121-13129. DOI: 10.1021/acs.est.5b01525.
[9] Bevelhimer M, Stewart A, Fortner A et al. CO2 is dominant greenhouse gas emitted from six hydropower reservoirs in southeastern United States during peak summer emissions. Water, 2016, 8(1): 15. DOI: 10.3390/w8010015.
[10] Miller BL, Arntzen EV, Goldman AE et al. Methane ebullition in temperate hydropower reservoirs and implications for US policy on greenhouse gas emissions. Environmental Management, 2017, 60(4): 615-629. DOI: 10.1007/s00267-017-0909-1.
[11] Wu Y, Mao XF, Xia LA et al. Microbial community abundance affects the methane ebullition flux in dahejia reservoir of the Yellow River in the warm season. Diversity, 2023, 15(2): 154. DOI: 10.3390/d15020154.
[12] Li Z, Zhang C, Liu L et al. Ebullition fluxes of CO2 and CH4 in Pengxi River, Three Gorges Reservoir. J Lake Sci, 2014, 26(5): 789-798. DOI:10.18307/2014.0518. [李哲, 张呈, 刘靓等. 三峡水库澎溪河CO2、CH4气泡释放通量初探. 湖泊科学, 2014, 26(5): 789-798.]
[13] Sturm K, Yuan Z, Gibbes B et al. Methane and nitrous oxide sources and emissions in a subtropical freshwater reservoir, South East Queensland, Australia. Biogeosciences, 2014, 11(18): 5245-5258. DOI: 10.5194/bg-11-5245-2014.
[14] Liu L, Yang ZJ, Delwiche K et al. Spatial and temporal variability of methane emissions from cascading reservoirs in the Upper Mekong River. Water Research, 2020, 186: 116319. DOI: 10.1016/j.watres.2020.116319.
[15] Keller M, Stallard RF. Methane emission by bubbling from Gatun Lake, Panama. Journal of Geophysical Research: Atmospheres, 1994, 99(D4): 8307-8319. DOI: 10.1029/92jd02170.
[16] Galy-Lacaux C, Delmas R, Jambert C et al. Gaseous emissions and oxygen consumption in hydroelectric dams: A case study in French Guyana. Global Biogeochemical Cycles, 1997, 11(4): 471-483. DOI: 10.1029/97gb01625.
[17] Duchemin E, Lucotte M, Canuel R et al. Comparison of greenhouse gas emissions from an old tropical reservoir with those from other reservoirs worldwide. SIL Proceedings, 1922-2010, 2000, 27(3): 1391-1395. DOI: 10.1080/03680770.1998.11901464.
[18] Joyce J. Physical controls on methane ebullition from reservoirs and lakes. Environmental and Engineering Geoscience, 2003, 9(2): 167-178. DOI: 10.2113/9.2.167.
[19] Tremblay A, Varfalvy L, Garneau M et al. Greenhouse gas Emissions-Fluxes and processes: Hydroelectric reservoirs and natural environments. Springer Science & Business Media, 2005.
[20] dos Santos MA, Rosa LP, Sikar B et al. Gross greenhouse gas fluxes from hydro-power reservoir compared to thermo-power plants. Energy Policy, 2006, 34(4): 481-488. DOI: 10.1016/j.enpol.2004.06.015.
[21] Bergier I, Novo EMLM, Ramos FM et al. Carbon dioxide and methane fluxes in the littoral zone of a tropical savanna reservoir (corumbá, Brazil). Oecologia Australis, 2011, 15(3): 666-681. DOI: 10.4257/oeco.2011.1503.17.
[22] DelSontro T, Kunz MJ, Kempter T et al. Spatial heterogeneity of methane ebullition in a large tropical reservoir. Environmental Science & Technology, 2011, 45(23): 9866-9873. DOI: 10.1021/es2005545.
[23] Marcon L, Sotiri K, Bleninger T et al. Acoustic mapping of gas stored in sediments of shallow aquatic systems linked to methane production and ebullition patterns. Frontiers in Environmental Science, 2022, 10: 876540. DOI: 10.3389/fenvs.2022.876540.
[24] Deshmukh C, Serca D, Delon C et al. Physical controls on CH4 emissions from a newly flooded subtropical freshwater hydroelectric reservoir: Nam Theun 2. Biogeosciences, 2014, 11(15): 4251-4269. DOI: 10.5194/bg-11-4251-2014.
[25] Wik M, Johnson JE, Crill PM et al. Sediment characteristics and methane ebullition in three subarctic lakes. Journal of Geophysical Research: Biogeosciences, 2018, 123(8): 2399-2411. DOI: 10.1029/2017jg004298.
[26] Zhu D, Wu Y, Chen H et al. Intense methane ebullition from open water area of a shallow peatland lake on the eastern Tibetan Plateau. Science of the Total Environment, 2016, 542: 57-64. DOI: 10.1016/j.scitotenv.2015.10.087.
[27] Yang GB, Zheng ZH, Abbott BW et al. Characteristics of methane emissions from alpine thermokarst lakes on the Tibetan Plateau. Nature Communications, 2023, 14(1): 3121. DOI: 10.1038/s41467-023-38907-6.
[28] Matveev A, Laurion I, Vincent WF. Methane and carbon dioxide emissions from thermokarst lakes on mineral soils. Arctic Science, 2018, 4(4): 584-604. DOI: 10.1139/as-2017-0047.
[29] Wik M, Crill PM, Bastviken D et al. Bubbles trapped in Arctic Lake ice: Potential implications for methane emissions. Journal of Geophysical Research, 2011, 116(G3): G03044. DOI: 10.1029/2011jg001761.
[30] Sepulveda-Jauregui A, Walter Anthony KM, Martinez-Cruz K et al. Methane and carbon dioxide emissions from 40 lakes along a north–south latitudinal transect in Alaska. Biogeosciences, 2015, 12(11): 3197-3223. DOI: 10.5194/bg-12-3197-2015.
[31] Strayer RF, Tiedje JM. In situ methane production in a small, hypereutrophic, hard-water lake: Loss of methane from sediments by vertical diffusion and ebullition. Limnology and Oceanography, 1978, 23(6): 1201-1206. DOI: 10.4319/lo.1978.23.6.1201.
[32] Mattson MD, Likens GE. Air pressure and methane fluxes. Nature, 1990, 347(6295): 718-719. DOI: 10.1038/347718b0.
[33] Casper P, Maberly SC, Hall GH et al. Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry, 2000, 49(1): 1-19.
[34] Bastviken D, Cole J, Pace M et al. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochemical Cycles, 2004, 18(4). DOI: 10.1029/2004gb002238.
[35] Martinez D, Anderson MA. Methane production and ebullition in a shallow, artificially aerated, eutrophic temperate lake (Lake Elsinore, CA). The Science of the Total Environment, 2013, 454/455: 457-465. DOI: 10.1016/j.scitotenv.2013.03.040.
[36] Bartosiewicz M, Laurion I, MacIntyre S. Greenhouse gas emission and storage in a small shallow lake. Hydrobiologia, 2015, 757(1): 101-115. DOI: 10.1007/s10750-015-2240-2.
[37] Schmiedeskamp M, Praetzel LSE, Bastviken D et al. Whole-lake methane emissions from two temperate shallow lakes with fluctuating water levels: Relevance of spatiotemporal patterns. Limnology and Oceanography, 2021, 66(6): 2455-2469. DOI: 10.1002/lno.11764.
[38] Sø JS, Sand-Jensen K, Martinsen KT et al. Methane and carbon dioxide fluxes at high spatiotemporal resolution from a small temperate lake. Science of the Total Environment, 2023, 878: 162895. DOI: 10.1016/j.scitotenv.2023.162895.
[39] Thottathil SD, Prairie YT. Prairie, Coupling of stable carbon isotopic signature of methane and ebullitive fluxes in northern temperate lakes. Science of the Total Environment, 2021, 777: 146117. DOI: 10.1016/j.scitotenv.2021.146117.
[40] Xiao QT, Zhang M, Hu ZH et al. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. Journal of Geophysical Research: Biogeosciences, 2017,122(7): 1597-1614. DOI: 10.1002/2017jg003805.
[41] Pu YN, Jia L, Yang SJ et al. The methane ebullition flux over algae zone of Lake Taihu. China Environmental Science, 2018, 38(10): 3914-3924. [蒲旖旎, 贾磊, 杨诗俊等. 太湖藻型湖区CH4冒泡通量. 中国环境科学, 2018. 38(10): 3914-3924.]
[42] Stepanenko VM, Mammarella I, Ojala A et al. LAKE 2.0: A model for temperature, methane, carbon dioxide and oxygen dynamics in lakes. Geoscientific Model Development, 2016, 9(5): 1977-2006. DOI: 10.5194/gmd-9-1977-2016.
[43] Hostetler SW, Bartlein PJ. Simulation of lake evaporation with application to modeling lake level variations of Harney-Malheur Lake, Oregon. Water Resources Research, 1990, 26(10): 2603-2612. DOI: 10.1029/wr026i010p02603.
[44] Fang X, Stefan HG. Temperature variability in lake sediments. Water Resources Research, 1998, 34(4): 717-729. DOI: 10.1029/97wr03517.
[45] Enríquez OR, Hummelink C, Bruggert GW et al. Growing bubbles in a slightly supersaturated liquid solution. Review of Scientific Instruments, 2013. 84(6). DOI: 10.1063/1.4810852.
[46] Boudreau BP. The physics of bubbles in surficial, soft, cohesive sediments. Marine and Petroleum Geology, 2012, 38(1): 1-18. DOI: 10.1016/j.marpetgeo.2012.07.002.
[47] Stepanenko VM, Machul’skaya EE, Glagolev MV et al. Numerical modeling of methane emissions from lakes in the permafrost zone. Izvestiya, Atmospheric and Oceanic Physics, 2011, 47(2): 252-264. DOI: 10.1134/s0001433811020113.
[48] Tan ZL, Zhuang QL, Walter Anthony K. Modeling methane emissions from Arctic Lakes: Model development and site-level study. Journal of Advances in Modeling Earth Systems, 2015, 7(2): 459-483. DOI: 10.1002/2014ms000344.
[49] Liang JH, McWilliams JC, Sullivan PP et al. Modeling bubbles and dissolved gases in the ocean. Journal of Geophysical Research: Oceans, 2011, 116(C3): C03015. DOI: 10.1029/2010jc006579.
| [1] |
Sanches LF, Guenet B, Marinho CC et al. Global regulation of methane emission from natural lakes. Scientific Reports, 2019, 9(1): 255. DOI:10.1038/s41598-018-36519-5 |
| [2] |
Rosentreter JA, Borges AV, Deemer BR et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nature Geoscience, 2021, 14(4): 225-230. |
| [3] |
Gerardo-Nieto O, Vega-Peñaranda A, Gonzalez-Valencia R et al. Continuous measurement of diffusive and ebullitive fluxes of methane in aquatic ecosystems by an open dynamic chamber method. Environmental Science & Technology, 2019, 53(9): 5159-5167. DOI:10.1021/acs.est.9b00425 |
| [4] |
Bastviken D, Tranvik LJ, Downing JA et al. Freshwater methane emissions offset the continental carbon sink. Science, 2011, 331(6013): 50. DOI:10.1126/science.1196808 |
| [5] |
McGinnis DF, Greinert J, Artemov Y et al. Fate of rising methane bubbles in stratified waters: How much methane reaches the atmosphere? Journal of Geophysical Research: Oceans, 2006, 111(C9). Journal of Geophysical Research: Oceans, 2006, 111(C9). DOI:10.1029/2005jc003183 |
| [6] |
DelSontro T, McGinnis DF, Sobek S et al. Extreme methane emissions from a Swiss hydropower reservoir: Contribution from bubbling sediments. Environmental Science & Technology, 2010, 44(7): 2419-2425. DOI:10.1021/es9031369 |
| [7] |
Xiao QT, Zhang M, Hu ZH et al. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. Journal of Geophysical Research: Biogeosciences, 2017, 122(7): 1597-1614. DOI:10.1002/2017jg003805 |
| [8] |
Pu YN, Jia L, Yang SJ et al. The methane ebullition flux over algae zone of Lake Taihu. China Environmental Science, 2018, 38(10): 3914-3924. [蒲旖旎, 贾磊, 杨诗俊等. 太湖藻型湖区CH4冒泡通量. 中国环境科学, 2018, 38(10): 3914-3924.] |
| [9] |
Walter KM, Zimov SA, Chanton JP et al. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature, 2006, 443(7107): 71-75. DOI:10.1038/nature05040 |
| [10] |
Johnson MS, Matthews E, Bastviken D et al. Spatiotemporal methane emission from global reservoirs. Journal of Geophysical Research: Biogeosciences, 2021, 126(8). DOI:10.1029/2021jg006305 |
| [11] |
Zheng YJ, Wu SA, Xiao SQ et al. Global methane and nitrous oxide emissions from inland waters and estuaries. Global Change Biology, 2022, 28(15): 4713-4725. DOI:10.1111/gcb.16233 |
| [12] |
Koschorreck M, Hentschel I, Boehrer B. Oxygen ebullition from lakes. Geophysical Research Letters, 2017, 44(18): 9372-9378. DOI:10.1002/2017gl074591 |
| [13] |
Aben RCH, Barros N, van Donk E et al. Cross continental increase in methane ebullition under climate change. Nature Communications, 2017, 8: 1682. DOI:10.1038/s41467-017-01535-y |
| [14] |
Davidson TA, Audet J, Jeppesen E et al. Synergy between nutrients and warming enhances methane ebullition from experimental lakes. Nature Climate Change, 2018, 8(2): 156-160. |
| [15] |
Linkhorst A, Paranaíba JR, Mendonça R et al. Spatially resolved measurements in tropical reservoirs reveal elevated methane ebullition at river inflows and at high productivity. Global Biogeochemical Cycles, 2021, 35(5). DOI:10.1029/2020gb006717 |
| [16] |
Wik M, Thornton BF, Bastviken D et al. Biased sampling of methane release from northern lakes: A problem for extrapolation. Geophysical Research Letters, 2016, 43(3): 1256-1262. DOI:10.1002/2015gl066501 |
| [17] |
Deemer BR, Harrison JA, Li SY et al. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. BioScience, 2016, 66(11): 949-964. DOI:10.1093/biosci/biw117 |
| [18] |
Kankaala P, Ojala A, Käki T. Temporal and spatial variation in methane emissions from a flooded transgression shore of a boreal lake. Biogeochemistry, 2004, 68(3): 297-311. DOI:10.1023/B:BIOG.0000031030.77498.1f |
| [19] |
Schubert CJ, Diem T, Eugster W. Methane emissions from a small wind shielded lake determined by eddy covariance, flux chambers, anchored funnels, and boundary model calculations: A comparison. Environmental Science & Technology, 2012, 46(8): 4515-4522. DOI:10.1021/es203465x |
| [20] |
Dück Y, Liu L, Lorke A et al. A novel freeze corer for characterization of methane bubbles and assessment of coring disturbances. Limnology and Oceanography: Methods, 2019, 17(5): 305-319. DOI:10.1002/lom3.10315 |
| [21] |
Noss C, Bodmer P, Koca K et al. Flow and turbulence driven water surface roughness and gas exchange velocity in streams. E3S Web of Conferences, 2018, 40: 05018. DOI:10.1051/e3sconf/20184005018 |
| [22] |
Ostrovsky I. The acoustic quantification of fish in the presence of methane bubbles in the stratified Lake Kinneret, Israel. Ices Journal of Marine Science, 2009, 66(6): 1043-1047. DOI:10.1093/icesjms/fsp103 |
| [23] |
Wilkinson J, Bodmer P, Lorke A. Methane dynamics and thermal response in impoundments of the Rhine River, Germany. Science of the Total Environment, 2019, 659: 1045-1057. DOI:10.1016/j.scitotenv.2018.12.424 |
| [24] |
Iwata H, Hirata R, Takahashi Y et al. Partitioning eddy-covariance methane fluxes from a shallow lake into diffusive and ebullitive fluxes. Boundary-Layer Meteorology, 2018, 169(3): 413-428. DOI:10.1007/s10546-018-0383-1 |
| [25] |
Walter KM, Engram M, Duguay CR et al. The potential use of synthetic aperture radar for estimating methane ebullition from Arctic Lakes. Journal of the American Water Resources Association, 2008, 44(2): 305-315. DOI:10.1111/j.1752-1688.2007.00163.x |
| [26] |
Engram M, Walter Anthony KM, Sachs T et al. Quantifying methane ebullition from northern lakes with space-borne synthetic aperture radar (SAR). AGU Fall Meeting, 2018. |
| [27] |
Engram M, Walter Anthony KM, Sachs T et al. Remote sensing northern lake methane ebullition. Nature Climate Change, 2020, 10(6): 511-517. DOI:10.1038/s41558-020-0762-8 |
| [28] |
Lindgren P, Grosse G, Meyer F et al. An object-based classification method to detect methane ebullition bubbles in early winter lake ice. Remote Sensing, 2019, 11(7): 822. DOI:10.3390/rs11070822 |
| [29] |
Varadharajan C, Hermosillo R, Hemond HF. A low-cost automated trap to measure bubbling gas fluxes. Limnology and Oceanography: Methods, 2010, 8: 363-375. DOI:10.4319/lom.2010.8.363 |
| [30] |
Delwiche K, Senft-Grupp S, Hemond H. A novel optical sensor designed to measure methane bubble sizesin situ. Limnology and Oceanography: Methods, 2015, 13(12): 712-721. DOI:10.1002/lom3.10060 |
| [31] |
Delwiche K, Hemond HF. An enhanced bubble size sensor for long-term ebullition studies. Limnology and Oceanography: Methods, 2017, 15(10): 821-835. DOI:10.1002/lom3.10201 |
| [32] |
Maher DT, Drexl M, Tait DR et al. iAMES: An inexpensive, Automated Methane Ebullition Sensor. Environmental Science & Technology, 2019, 53(11): 6420-6426. DOI:10.1021/acs.est.9b01881 |
| [33] |
Thanh Duc N, Silverstein S, Wik M et al. Technical note: Greenhouse gas flux studies: An automated online system for gas emission measurements in aquatic environments. Hydrology and Earth System Sciences, 2020, 24(7): 3417-3430. DOI:10.5194/hess-24-3417-2020 |
| [34] |
Ostrovsky I, McGinnis DF, Lapidus L et al. Quantifying gas ebullition with echosounder: The role of methane transport by bubbles in a medium-sized lake. Limnology and Oceanography: Methods, 2008, 6: 105-118. |
| [35] |
Linkhorst A, Hiller C, DelSontro T et al. Comparing methane ebullition variability across space and time in a Brazilian Reservoir. Limnology and Oceanography, 2019, 65: 1623-1634. DOI:10.1002/lno.11410 |
| [36] |
Wik M, Crill PM, Varner RK et al. Multiyear measurements of ebullitive methane flux from three subarctic lakes. Journal of Geophysical Research: Biogeosciences, 2013, 118(3): 1307-1321. DOI:10.1002/jgrg.20103 |
| [37] |
Tušer M, Picek T, Sajdlová Z et al. Seasonal and spatial dynamics of gas ebullition in a temperate water-storage reservoir. Water Resources Research, 2017, 53(10): 8266-8276. DOI:10.1002/2017wr020694 |
| [38] |
Marcon L, Bleninger T, Mannich M et al. High-frequency measurements of gas ebullition in a Brazilian subtropical reservoiridentification of relevant triggers and seasonal patterns. Environmental Monitoring and Assessment, 2019, 191(6): 357. DOI:10.1007/s10661-019-7498-9 |
| [39] |
Li Z, Zhang C, Liu L et al. Ebullition fluxes of CO2 and CH4 in Pengxi River, Three Gorges Reservoir. J Lake Sci, 2014, 26(5): 789-798. [李哲, 张呈, 刘靓等. 三峡水库澎溪河CO2、CH4气泡释放通量初探. 湖泊科学, 2014, 26(5): 789-798. DOI:10.18307/2014.0518] |
| [40] |
Walter KM, Chanton JP, Chapin FS III et al. Methane production and bubble emissions from Arctic Lakes: Isotopic implications for source pathways and ages. Journal of Geophysical Research: Biogeosciences, 2008, 113(G3). DOI:10.1029/2007jg000569 |
| [41] |
Maeck A, Hofmann H, Lorke A. Pumping methane out of aquatic sediments-ebullition forcing mechanisms in an impounded river. Biogeosciences, 2014, 11(11): 2925-2938. DOI:10.5194/bg-11-2925-2014 |
| [42] |
Liu L, Yang ZJ, Delwiche K et al. Spatial and temporal variability of methane emissions from cascading reservoirs in the Upper Mekong River. Water Research, 2020, 186: 116319. DOI:10.1016/j.watres.2020.116319 |
| [43] |
Wu Y, Mao XF, Xia LA et al. Microbial community abundance affects the methane ebullition flux in dahejia reservoir of the Yellow River in the warm season. Diversity, 2023, 15(2): 154. DOI:10.3390/d15020154 |
| [44] |
Eugster W, DelSontro T, Shaver GR et al. Interannual, summer, and diel variability of CH4 and CO2 effluxes from Toolik Lake, Alaska, during the ice-free periods 2010-2015. Environmental Science Processes & Impacts, 2020, 22(11): 2181-2198. DOI:10.1039/d0em00125b |
| [45] |
Keller M, Stallard RF. Methane emission by bubbling from Gatun Lake, Panama. Journal of Geophysical Research: Atmospheres, 1994, 99(D4): 8307-8319. DOI:10.1029/92jd02170 |
| [46] |
Iwata H, Nakazawa K, Sato H et al. Temporal and spatial variations in methane emissions from the littoral zone of a shallow mid-latitude lake with steady methane bubble emission areas. Agricultural and Forest Meteorology, 2020, 295: 108184. DOI:10.1016/j.agrformet.2020.108184 |
| [47] |
Deshmukh C, Serca D, Delon C et al. Physical controls on CH4 emissions from a newly flooded subtropical freshwater hydroelectric reservoir: Nam Theun 2. Biogeosciences, 2014, 11(15): 4251-4269. DOI:10.5194/bg-11-4251-2014 |
| [48] |
Harrison JA, Deemer BR, Birchfield MK et al. Reservoir water-level drawdowns accelerate and amplify methane emission. Environmental Science & Technology, 2017, 51(3): 1267-1277. DOI:10.1021/acs.est.6b03185 |
| [49] |
Liu L, Sotiri K, Dück Y et al. The control of sediment gas accumulation on spatial distribution of ebullition in Lake Kinneret. Geo-Marine Letters, 2020, 40(4): 453-466. DOI:10.1007/s00367-019-00612-z |
| [50] |
Wilkinson J, Maeck A, Alshboul Z et al. Continuous seasonal river Ebullition measurements linked to sediment methane formation. Environmental Science & Technology, 2015, 49(22): 13121-13129. DOI:10.1021/acs.est.5b01525 |
| [51] |
Schmiedeskamp M, Praetzel LSE, Bastviken D et al. Whole-lake methane emissions from two temperate shallow lakes with fluctuating water levels: Relevance of spatiotemporal patterns. Limnology and Oceanography, 2021, 66(6): 2455-2469. DOI:10.1002/lno.11764 |
| [52] |
Xiao SB, Liu DF, Wang YC et al. Temporal variation of methane flux from Xiangxi Bay of the Three Gorges Reservoir. Scientific Reports, 2013, 3: 2500. DOI:10.1038/srep02500 |
| [53] |
Greene S, Walter Anthony KM, Archer D et al. Modeling the impediment of methane ebullition bubbles by seasonal lake ice. Biogeosciences, 2014, 11(23): 6791-6811. DOI:10.5194/bg-11-6791-2014 |
| [54] |
Wik M, Johnson JE, Crill PM et al. Sediment characteristics and methane ebullition in three subarctic lakes. Journal of Geophysical Research: Biogeosciences, 2018, 123(8): 2399-2411. DOI:10.1029/2017jg004298 |
| [55] |
DelSontro T, Boutet L, St-Pierre A et al. Methane ebullition and diffusion from northern ponds and lakes regulated by the interaction between temperature and system productivity. Limnology and Oceanography, 2016, 61: S62-S77. DOI:10.1002/lno.10335 |
| [56] |
Wik M, Thornton BF, Bastviken D et al. Energy input is primary controller of methane bubbling in subarctic lakes. Geophysical Research Letters, 2014, 41(2): 555-560. DOI:10.1002/2013gl058510 |
| [57] |
Quadra GR, Sobek S, Paranaíba JR et al. High organic carbon burial but high potential for methane ebullition in the sediments of an Amazonian hydroelectric reservoir. Biogeosciences, 2020, 17(6): 1495-1505. DOI:10.5194/bg-17-1495-2020 |
| [58] |
Barros N, Cole JJ, Tranvik LJ et al. Carbon emission from hydroelectric reservoirs linked to reservoir age and latitude. Nature Geoscience, 2011, 4(9): 593-596. DOI:10.1038/ngeo1211 |
| [59] |
Sturm K, Yuan Z, Gibbes B et al. Methane and nitrous oxide sources and emissions in a subtropical freshwater reservoir, South East Queensland, Australia. Biogeosciences, 2014, 11(18): 5245-5258. DOI:10.5194/bg-11-5245-2014 |
| [60] |
Beaulieu JJ, McManus MG, Nietch CT. Estimates of reservoir methane emissions based on a spatially balanced probabilistic-survey. Limnology and Oceanography, 2016, 61(S1). DOI:10.1002/lno.10284 |
| [61] |
Zhao Y, Zeng Y, Wu BF et al. Review of methods for measuring greenhouse gas flux from the air-water interface of reservoirs. Advances in Water Science, 2011, 22(1): 135-146. [赵炎, 曾源, 吴炳芳等. 水库水气界面温室气体通量监测方法综述. 水科学进展, 2011, 22(1): 135-146.] |
| [62] |
Rosa LP, Dos Santos MA, Matvienko B et al. Biogenic gas production from major Amazon Reservoirs, Brazil. Hydrological Processes, 2003, 17(7): 1443-1450. DOI:10.1002/hyp.1295 |
| [63] |
Flury S, McGinnis DF, Gessner MO. Methane emissions from a freshwater marsh in response to experimentally simulated global warming and nitrogen enrichment. Journal of Geophysical Research-Biogeosciences, 2010, 115. DOI:10.1029/2009jg001079 |
| [64] |
DelSontro T, McGinnis DF, Wehrli B et al. Size does matter: Importance of large bubbles and small-scale hot spots for methane transport. Environmental Science & Technology, 2015, 49(3): 1268-1276. DOI:10.1021/es5054286 |
| [65] |
Maeck A, DelSontro T, McGinnis D et al. Sediment trapping by dams creates methane emission hot spots. Environmental Science & Technology, 2013, 47(15): 8130-8137. DOI:10.1021/es4003907 |
| [66] |
Huang Y, Yasarer LMW, Li Z et al. Air-water CO2 and CH4 fluxes along a river-reservoir continuum: Case study in the Pengxi River, a tributary of the Yangtze River in the Three Gorges Reservoir, China. Environmental Monitoring and Assessment, 2017, 189(5): 1-15. DOI:10.1007/s10661-017-5926-2 |
| [67] |
Miller BL, Arntzen EV, Goldman AE et al. Methane ebullition in temperate hydropower reservoirs and implications for US policy on greenhouse gas emissions. Environmental Management, 2017, 60(4): 615-629. DOI:10.1007/s00267-017-0909-1 |
| [68] |
Walter KM, Smith LC, Chapin FS Ⅲ. Methane bubbling from northern lakes: Present and future contributions to the global methane budget. Philosophical Transactions Series A, Mathematical, Physical, and Engineering Sciences, 2007, 365(1856): 1657-1676. DOI:10.1098/rsta.2007.2036 |
| [69] |
Zhu D, Wu Y, Chen H et al. Intense methane ebullition from open water area of a shallow peatland lake on the eastern Tibetan Plateau. Science of the Total Environment, 2016, 542: 57-64. DOI:10.1016/j.scitotenv.2015.10.087 |
| [70] |
Zhang L, Xia XH, Liu SD et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai-Tibet Plateau. Nature Geoscience, 2020, 13(5): 349-354. DOI:10.1038/s41561-020-0571-8 |
| [71] |
Yang P, Tong C. Emission paths and measurement methods for greenhouse gas fluxes from freshwater ecosystems: A review. Acta Ecologica Sinica, 2015, 35(20): 6868-6880. [杨平, 仝川. 淡水水生生态系统温室气体排放的主要途径及影响因素研究进展. 生态学报, 2015, 35(20): 6868-6880. DOI:10.5846/stxb201406231298] |
| [72] |
Langenegger T, Vachon D, Donis D et al. What the bubble knows: Lake methane dynamics revealed by sediment gas bubble composition. Limnology and Oceanography, 2019, 64(4): 1526-1544. DOI:10.1002/lno.11133 |
| [73] |
Schmid M, Ostrovsky I, McGinnis DF. Role of gas ebullition in the methane budget of a deep subtropical lake: What can we learn from process-based modeling?. Limnology and Oceanography, 2017, 62(6): 2674-2698. DOI:10.1002/lno.10598 |
| [74] |
van Kessel T, van Kesteren WGM. Gas production and transport in artificial sludge depots. Waste Management, 2002, 22(1): 19-28. DOI:10.1016/s0956-053x(01)00021-6 |
| [75] |
Tokida T, Miyazaki T, Mizoguchi M. Physical controls on ebullition losses of methane from peatlands. Carbon Cycling in Northern Peatlands, 2013, 219-228. DOI:10.1029/2008gm000805 |
| [76] |
Enríquez OR, Hummelink C, Bruggert GW et al. Growing bubbles in a slightly supersaturated liquid solution. Review of Scientific Instruments, 2013, 84(6). DOI:10.1063/1.4810852 |
| [77] |
Boudreau BP. The physics of bubbles in surficial, soft, cohesive sediments. Marine and Petroleum Geology, 2012, 38(1): 1-18. DOI:10.1016/j.marpetgeo.2012.07.002 |
| [78] |
Chen X, Slater L. Methane emission through ebullition from an estuarine mudflat: 1. A conceptual model to explain tidal forcing based on effective stress changes. Water Resources Research, 2016, 52(6): 4469-4485. DOI:10.1002/2015wr018058 |
| [79] |
Gardiner B, Boudreau B, Johnson B. Growth of disk-shaped bubbles in sediments. Geochimica et Cosmochimica Acta, 2003, 67(8): 1485-1494. DOI:10.1016/S0016-7037(02)01072-4 |
| [80] |
Algar CK, Boudreau BP. Transient growth of an isolated bubble in muddy, fine-grained sediments. Geochimica et Cosmochimica Acta, 2009, 73(9): 2581-2591. DOI:10.1016/j.gca.2009.02.008 |
| [81] |
Liu L, De Kock T, Wilkinson J et al. Methane bubble growth and migration in aquatic sediments observed by X-ray μCT. Environmental Science & Technology, 2018, 52(4): 2007-2015. DOI:10.1021/acs.est.7b06061 |
| [82] |
Liu L, Wilkinson J, Koca K et al. The role of sediment structure in gas bubble storage and release. Journal of Geophysical Research-Biogeosciences, 2016, 121(7): 1992-2005. DOI:10.1002/2016jg003456 |
| [83] |
Jain A, Juanes R. Preferential Mode of gas invasion in sediments: Grain-scale mechanistic model of coupled multiphase fluid flow and sediment mechanics. Journal of Geophysical Research: Solid Earth, 2009, 114(B8). DOI:10.1029/2008jb006002 |
| [84] |
Wheeler SJ. A conceptual model for soils containing large gas bubbles. Geotechnique, 1988, 38(3): 389-397. |
| [85] |
Algar CK, Boudreau B. Stability of bubbles in a linear elastic medium: Implications for bubble growth in marine sediments. Journal of Geophysical Research: Earth Surface, 2010, 115(F3). DOI:10.1029/2009jf001312 |
| [86] |
Katsman R, Ostrovsky I, Makovsky Y. Methane bubble growth in fine-grained muddy aquatic sediment: Insight from modeling. Earth and Planetary Science Letters, 2013, 377/378: 336-346. DOI:10.1016/j.epsl.2013.07.011 |
| [87] |
Mahabadi N, Zheng XL, Yun TS et al. Gas bubble migration and trapping in porous media: Pore-scale simulation. Journal of Geophysical Research: Solid Earth, 2018, 123(2): 1060-1071. DOI:10.1002/2017jb015331 |
| [88] |
Algar CK, Boudreau BP, Barry MA. Initial rise of bubbles in cohesive sediments by a process of viscoelastic fracture. Journal of Geophysical Research, 2011, 116(B4): B04207. DOI:10.1029/2010jb008133 |
| [89] |
Algar CK, Boudreau BP, Barry MA. Release of multiple bubbles from cohesive sediments. Geophysical Research Letters, 2011, 38(8). DOI:10.1029/2011gl046870 |
| [90] |
Shagapov VS, Chiglinseva AS, Rusinov AA et al. On the migration of a single gas bubble in water. High Temperature, 2017, 55: 420-425. DOI:10.1134/S0018151X17020171 |
| [91] |
Warzinski RP, Lynn R, Haljasmaa I et al. Dynamic morphology of gas hydrate on a methane bubble in water: Observations and new insights for hydrate film models. Geophysical Research Letters, 2014, 41(19): 6841-6847. DOI:10.1002/2014gl061665 |
| [92] |
Sauter EJ, Muyakshin SI, Charlou JL et al. Methane discharge from a deep-sea submarine mud volcano into the upper water column by gas hydrate-coated methane bubbles. Earth and Planetary Science Letters, 2006, 243(3/4): 354-365. DOI:10.1016/j.epsl.2006.01.041 |
| [93] |
Huttunen J, Lappalainen K, Saarijarvi E et al. A novel sediment gas sampler and a subsurface gas collector used for measurement of the ebullition of methane and carbon dioxide from a eutrophied lake. Science of the Total Environment, 2001, 266(1/2/3): 153-158. DOI:10.1016/s0048-9697(00)00749-x |
| [94] |
Darmana D, Deen NG, Kuipers JAM. Detailed modeling of hydrodynamics, mass transfer and chemical reactions in a bubble column using a discrete bubble model. Chemical Engineering Science, 2005, 60(12): 3383-3404. DOI:10.1016/j.ces.2005.01.025 |
| [95] |
Lau YM, Bai W, Deen NG et al. Numerical study of bubble break-up in bubbly flows using a deterministic Euler-Lagrange framework. Chemical Engineering Science, 2014, 108: 9-22. DOI:10.1016/j.ces.2013.12.034 |
| [96] |
Battistella A, Aelen S, Roghair I et al. Euler-lagrange modeling of bubbles formation in supersaturated water. Chem Engineering, 2018, 2(3): 39. DOI:10.3390/chemengineering2030039 |
| [97] |
Joyce J. Physical controls on methane ebullition from reservoirs and lakes. Environmental and Engineering Geoscience, 2003, 9(2): 167-178. DOI:10.2113/9.2.167 |
| [98] |
Marcon L, Sotiri K, Bleninger T et al. Acoustic mapping of gas stored in sediments of shallow aquatic systems linked to methane production and ebullition patterns. Frontiers in Environmental Science, 2022, 10: 876540. DOI:10.3389/fenvs.2022.876540 |
| [99] |
Tan ZL, Zhuang QL, Walter Anthony K. Modeling methane emissions from Arctic Lakes: Model development and site-level study. Journal of Advances in Modeling Earth Systems, 2015, 7(2): 459-483. DOI:10.1002/2014ms000344 |
| [100] |
Guo MY, Zhuang QL, Tan ZL et al. Rising methane emissions from boreal lakes due to increasing ice-free days. Environmental Research Letters, 2020, 15(6): 064008. |
| [101] |
Tan ZL, Zhuang QL, Shurpali NJ et al. Modeling CO2 emissions from Arctic Lakes: Model development and site-level study. Journal of Advances in Modeling Earth Systems, 2017, 9(5): 2190-2213. DOI:10.1002/2017ms001028 |
| [102] |
Stepanenko VM, Mammarella I, Ojala A et al. LAKE 2.0: A model for temperature, methane, carbon dioxide and oxygen dynamics in lakes. Geoscientific Model Development, 2016, 9(5): 1977-2006. DOI:10.5194/gmd-9-1977-2016 |
| [103] |
Stepanenko VM, Machul'skaya EE, Glagolev MV et al. Numerical modeling of methane emissions from lakes in the permafrost zone. Izvestiya, Atmospheric and Oceanic Physics, 2011, 47(2): 252-264. DOI:10.1134/s0001433811020113 |
 2024, Vol. 36
2024, Vol. 36 

