(2: 中国科学院亚热带农业生态研究所, 中国科学院洞庭湖湿地生态系统观测研究站, 长沙 410125)
(3: 中国科学院大学, 北京 100049)
(4: 中国气象局国家气候中心, 北京 100081)
(2: Dongting Lake Station for Wetland Ecosystem Research, Institute of Subtropical Agriculture, Chinese Academy of Sciences, Changsha 410125, P. R. China)
(3: University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
(4: National Climate Center, China Meteorological Administration, Beijing 100081, P. R. China)
甲烷(Methane, CH4)是导致全球增温的重要温室气体之一,在百年尺度上其单分子增温潜势是二氧化碳(CO2)的28倍[1].湿地是最大的CH4自然排放源(153~227 Tg/a),占全球自然CH4平均年排放量的62 % [2].当前,全球湿地CH4的排放过程及机理已得到广泛关注,但整体而言,高纬度泥炭地和滨海湿地开展较多,而内陆湖泊湿地的研究仍较少,尤其是中低纬度地区[3].事实上,中低纬度内陆湖泊湿地由于水热资源丰富,物质生产量高,其淹水过程引发的厌氧环境为CH4产生创造了先决条件,是大气CH4的重要排放源[4].因此,研究内陆湖泊生态系统的CH4排放对完善国家温室气体清单以及未来全球陆地CH4的收支估算有着重要现实意义.
湿地水体CH4传输主要有两种途径:一是气泡传输(ebullitive transport),即底泥产生的CH4在水体中累积,当水体CH4浓度足够高时产生气体压力,从而形成含CH4的气泡,在水面破裂而排放到大气[5-6];二是扩散传输(diffusive transport),即随水中浓度梯度分子扩散排出[7-8]. CH4通过气泡排放的情况一般出现在浅水淡水生态系统中[9].较扩散传输而言,气泡传输速度快,CH4排放量大.而CH4气泡排放和扩散排放对CH4总排放的贡献度在不同类型水体差异较大,Casper等[10]在英国北部湖泊研究中发现96 %的CH4通过气泡排放.在西伯利亚泥炭地湖泊研究中,CH4气泡排放通量仅占CH4总排放通量的11 % ~40 %,而在池塘中未观测到CH4气泡排放[11]. Delsontro等[9]研究发现加拿大浅水湖泊中CH4气泡排放仅占湖泊CH4总排放的18 % ~22 %,在池塘中,气泡排放占水体CH4总排放的56 %.而在中低纬度内陆湖泊、湿地,气泡排放和扩散排放在CH4排放中的贡献目前缺乏应有的关注.
一般而言,CH4的产生和排放过程由复杂的微生物群体调控,受多种因素,如含氧量、pH、电导率、可利用性有机质、水温和水位变化等的影响[12-14].有研究表明,温度在有机质矿化和CH4产生过程中起着主导作用[15]. CH4气泡排放和扩散排放在CH4总排放中的贡献度与水温密切相关[9].随着水体温度上升,产甲烷菌活性增强导致产CH4量增加,从而使水体迅速达到CH4过饱和状态,最终促进CH4气泡排放[6].但就CH4扩散传输而言,水温上升在增加CH4产率[16]的同时可能大幅增加CH4氧化速率[17],使大量CH4在排放到大气前被氧化,从而降低了CH4排放.当前,就中低纬度内陆湖泊湿地而言,影响CH4气泡传输和扩散传输的关键环境因子仍不清楚.
本文以洞庭湖为研究对象,在湿地洲滩沿高程梯度选择3种典型生境类型:光滩地,无植被覆盖;苔草地,主要物种为苔草(Carex spp.);芦苇地,主要物种为南荻(Triarrhena lutarioriparia)和芦苇(Phragmites australis).研究洪水期(6 -11月)不同生境类型CH4的扩散和气泡排放通量,在监测CH4通量的同时同步测定水体理化指标,分析CH4排放通量与环境因子之间的相关性.本文旨在估算洞庭湖湿地洪水期CH4气泡排放和扩散排放通量在CH4总排放中的相对贡献;探明影响CH4气泡排放和扩散排放通量的关键环境因子.
1 材料与方法 1.1 研究区介绍洞庭湖(28°30′~30°20′N, 111°40′~113°10′E)湖泊面积2625 km2,是我国第二大淡水湖.洞庭湖区属北亚热带湿润季风气候,年平均气温为17℃,其中最冷月(1月)平均气温为7.7℃,最热月(7月)平均气温为32.1℃,具有四季分明,热量丰富,降水充沛,降水季节集中,降水量年际变化大等鲜明特点.洞庭湖也是我国水位变幅最大的内陆湖泊,水位年内变化幅度多年平均值为3.50 m(小河咀)~10.01 m(城陵矶)[18].洞庭湖流域降水量年内分配极不均匀,整体呈现“抛物线”型分配模式,每年6 -10月为洪汛期,洲滩淹没时间长达5个月[19].
1.2 样点布设方案本研究样地位于中国科学院洞庭湖湿地生态系统观测研究站(CERN站)长期观测样地内,样地面积2 km2,从水面到大堤沿高程梯度主要分布着光滩(无植被覆盖)、苔草群落和芦苇群落3种典型生境(图 1).由于洞庭湖湿地CH4排放主要集中在洪水期[20],所以本研究的采样时间段设为2017年7月12日-11月4日,该时间段为洞庭湖湿地洲滩淹没期(洪水期).采样方法为样带法,即:每次采样时,在每个生境中平行于大堤设置一条长度200 m的样带,在样带上每隔50 m设置1个漂浮箱用于CH4通量的观测,每个生境设置4个漂浮箱作为4次重复.根据采样区天气情况,采样周期设为每10~15天一次.由于芦苇样地高程较高(28 m,黄海高程),淹水时间较短,且部分高程较高地段芦苇处于半淹状态,故在芦苇样地中仅在7月12日、7月27日和10月17日进行采样,且芦苇地通量观测未包含露出水面植被的CH4排放.
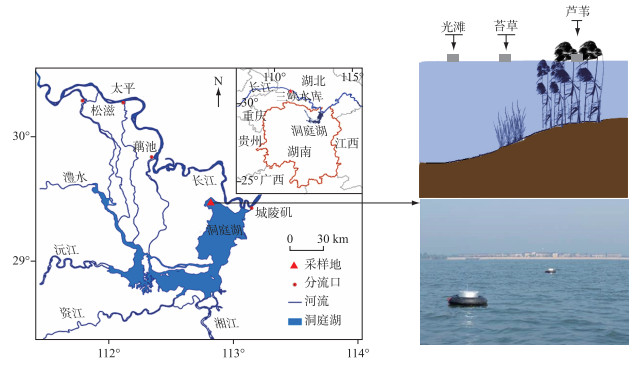
|
图 1 洞庭湖湿地样点布设示意 Fig.1 Schematic diagram of sampling sites in Lake Dongting wetlands |
水体CH4总排放通量(Ftotal)采用漂浮箱法测定.由于漂浮箱法能够收集扩散排放和气泡排放的CH4,被广泛用于水体CH4通量观测.在每个样方设置1个漂浮暗箱(箱体由PVC管制作,底面直径40 cm,高30 cm,体积0.038 m3),将其放入充气轮胎中使其漂浮在水面上.在箱体下水采样之前,先使箱内气体与大气自由混合,然后轻轻倒扣在水面上,操作过程中尽量减少对水面的扰动,以免影响测量结果.气体采集期间漂浮箱为密封状态,利用医用注射器通过三通阀从漂浮箱中取30 ml气体注入色谱气瓶(Labco, UK)内,每间隔5 min采集一个箱内气体样品,每个漂浮箱采集5次,采集气体样品的同时测定箱内气温,采集的气样带回实验室用配备有火焰离子化检测器(FID)和电子捕获检测器(ECD)的气相色谱仪(Agilent 7890A,USA)测量,气体通量由下式计算:
| $ F_{\text { total }}=k \cdot V / A(\mathrm{d} C / \mathrm{d} t) \rho\left[T_{0} /\left(T_{0}+T_{1}\right)\right] p / p_{0} $ | (1) |
式中,ρ为CH4密度(g/L),V为采样箱气室容积(m3),A为箱体与水面的接触面积(m2),dC/dt为漂浮箱气室内CH4气体的初始浓度变化率(μl/(L ·min));T1为采样箱气室内的温度(℃);p为采样时的大气压力(hPa);T0和p0分别为标准状况下的气温(273 K)及大气压力(1013 hPa);k为量纲换算系数.
dC/dt根据采集的5个气体样品中的CH4浓度来计算,优先采用非线性拟合方法,其次是线性拟合方法.采用非线性算法估算CH4通量时,气体浓度需同时满足以下条件:(1)5次浓度观测数据全部有效(n=5);(2)非线性相关系数(RNL)大于0.87;(3)RNL和线性相关系数(RL)之差(RNL- RL)大于0.001;(4)非线性拟合给出的采样箱密闭阶段初始气体浓度时间变化率大于线性拟合给出的采样箱密闭阶段初始气体浓度时间变化率.当以上条件均不符合时,采用线性拟合方法估算CH4通量.其质控标准为:5次观测浓度全部都有效(n=5)时,RL > 0.87;只有4次观测值有效(n=4)时,RL > 0.95;只有3次观测值有效(n=3)时,RL > 0.996[21].
1.3.2 CH4扩散通量测定方法水体CH4扩散排放通量(Fdiff)由扩散模型法测定.扩散模型法主要是依据水-气界面的气体交换,由气体扩散速率和浓度梯度共同作用的原理建立[22],它克服了水体流动和风速对测定结果的影响[23].当前主要的模型有LM86[24]、W92a、W92b[22]和RC01[25]等,不同模型算出的CH4通量有很大的差异[26-29].高洁等[29]认为:对于没有气泡排放的水体,RC01、W92a和W92b模型的平均值能较好地代表CH4扩散通量,因此在本文中选择W92a、W92b和RC01模型的平均值来代表洞庭湖水体CH4扩散通量.
水体CH4浓度测定.依据Casper等[30]的方法,准备清洗干净的100 ml玻璃厌氧瓶,采样前先称好30 g氯化钠装入盐水瓶中,随后盖上密封塞并将厌氧瓶抽真空(真空度约为-88 kPa).在漂浮箱观测CH4通量的同时,在采样点附近采集表层和底层的水样,用60 ml的医用塑料注射器从取水器中抽取50 ml水样注入真空盐水瓶中,晃动盐水瓶使溶解在水中的CH4释放到盐水瓶上部空间然后尽快将盐水瓶运输回实验室.将盐水瓶带回实验室后,用氦气平衡盐水瓶内气压,随后用30 ml的医用塑料注射器从盐水瓶上部空间抽取15 ml气样注入色谱气瓶中(Labco, UK),随后上气相色谱仪分析其CH4浓度.最终水体溶解的CH4浓度(Cobs)计算公式为:
| $ C_{\mathrm{obs}}=C_{0} \cdot V_{0} / V_{1} $ | (2) |
式中,C0为盐水瓶上部空间CH4浓度(μmol/L),V0为盐水瓶上部空间体积(L),V1为采集的水样体积(L).
CH4扩散通量(Fdiff)的计算公式为:
| $ F_{\mathrm{diff}}=c \cdot K_{\mathrm{w}}\left(C_{\mathrm{obs}}-C_{\mathrm{eq}}\right) $ | (3) |
式中,c为量纲转换系数,Kw为气体在水-气界面逆浓度梯度扩散的速度(cm/s); 双层模型的Kw值定义为风速和气体Schmidt(简称Sc)的函数,其中Sc是水的动力黏度与待测气体分子扩散速率之比,Wanninkhof[21]给出了水体中CH4的Sc与0~30℃范围水温(T)的关系式:
| $ S_{\mathrm{e}}\left(C H_{4}\right)=2309.2-120.31 T+3.4209 T^{2}-0.040437 T^{3} $ | (4) |
不同风速条件下Kw随Sc和风速变化的公式如表 1,本研究风速数据来源于实验样地通量塔数据.
| 表 1 与风速有关的气体交换估算公式 Tab. 1 Gas transfer velocity formulas depend on wind speed |
式(3)中,Ceq为表层水体与大气达平衡时的水中气体浓度(μmol/L),实际水温条件下CH4的Ceq值用Henry定律计算,即:
| $ C_{\mathrm{eq}}=K_{\mathrm{H}} \cdot P_{\mathrm{A}} $ | (5) |
式中,PA为CH4的大气分压;KH为采样时水文条件下的气体Henry常数(μmol/(L ·atm)),其值依据Henry定律和Sander(1999, http://www.mpch-mainz.mpg.de/~sander/res.henty. [1999-04-08]),根据实际水温计算确定:
| $ {K_{\rm{H}}} = K_{\rm{H}}^\theta \cdot {{\rm{e}}^{{K_{\rm{T}}}\left( {\frac{1}{{{T_K}}} - \frac{1}{{{T^\theta }}}} \right)}} $ | (6) |
式中,Tθ=298 K,表示常温;KHθ=1308 μmol/(L ·atm),为298 K和1 atm条件下的Henry常数;KT=2675,为表征Henry常数随温度变化的参数;TK为实际绝对温度(K).
1.3.3 CH4气泡排放通量计算气泡排放通量(Febul)计算公式为:
| $ F_{\text { ebul }}=F_{\text { total }}-F_{\text { diff }} $ | (7) |
取水器采集的部分水样用于水体CH4浓度分析,另外取500 ml水样装入聚四氟乙烯采样瓶带回实验室用于水体理化指标的分析.同时用YSI多参数水质仪(6600V2,USA)现场测定水温(T)、pH、溶解氧(DO)、水位、电导率、氧化还原电位和有色可溶性有机物(CDOM)等指标.采用流动注射仪AA3(SEAL, Germany)测定水体的铵态氮(NH4+-N)、硝态氮(NO3--N)以及用碱性过硫酸钾溶液消解后的总氮(TN)和总磷(TP)浓度;用TOC分析仪(Shimadzu, Japan)测定水体总有机碳(TOC)和可溶性有机碳(DOC)浓度.
1.5 数据分析方法所有数据采用Microsoft Excel 2016软件进行实验数据预处理,利用SPSS 16.0进行统计分析.采用双因素方差分析(Two-way ANOVA)检验水体环境因子以及水体CH4浓度在不同生境以及不同水体层中的差异;采用多重比较LSD法检验相同水层内不同生境的水体环境因子的差异;数据分析前,对数据进行正态性检验(P-P plot)和方差齐次性检验(Levene's test).通过逐步回归分析(Stepwise Regression Analysis)确定影响CH4总排放通量、扩散排放通量、气泡排放通量的关键水体环境因子(pH、T、DO、氧化还原电位、TOC、DOC、TN、TP、NH4+-N、NO3--N等);采用线性回归模型(Linear Regression Model)表征CH4总排放通量、气泡排放通量、扩散排放通量和水体关键环境因子间的关系.图表制作和曲线拟合采用Origin 8.0软件(OriginLab Ltd, USA)完成.
2 结果 2.1 水体环境变化对不同生境和不同水层水体环境因子的方差分析表明:总体来看,表层水体溶解氧和pH显著高于底层(P < 0.05,表 2),CDOM和电导率显著低于底层(P < 0.05,表 2),其他环境因子差异均不显著(P>0.05,表 2).从光滩到芦苇,随着高程增加,表层水体溶解氧、pH逐渐降低,而电导率、TN和TP浓度显著升高(P < 0.05,表 2);而底层水体TOC浓度在光滩和苔草地最高,分别为4.79±0.16和4.58±0.13 mg/L,但TN和TP浓度在芦苇地最高,分别为1.41±0.08和0.13±0.01 mg/L(P < 0.05,表 2).
| 表 2 不同生境不同水层的水体环境特征 Tab. 2 Water environmental properties in sampling sites and water layers |
本实验测得的水体CH4浓度介于0~4.17 μmol/L之间,在洪水期第1次采样(7月12日),光滩、苔草和芦苇的表层水体CH4浓度分别为0.09±0.008、0.12±0.021和0.11±0.013 μmol/L,底层水体CH4浓度分别为4.17±2.10、2.57±1.60和0.52±0.14 μmol/L,3个生境底层水体CH4浓度均显著高于表层水体CH4浓度(P < 0.01,图 2),随着淹水时间增加,表层和底层的水体CH4浓度差异减少,到淹水后期差异不显著(P>0.05,图 2).
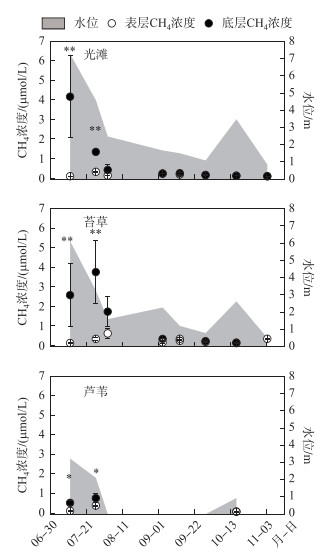
|
图 2 3种生境不同水层的水体CH4浓度变化 (*表示水体表层与底层CH4浓度差异, *P < 0.05,** P < 0.01) Fig.2 Variation of methane concentration of different water layers in three sampling sites |
利用漂浮箱法在光滩、苔草和芦苇3个生境测定的CH4 Ftotal分别介于0.28~12.88、0.28~22.68和0.080~16.24 mg (C)/(m2 ·h)之间,其中洪水期苔草地的CH4 Ftotal均值为6.49±3.12 mg (C)/(m2 ·h),高于光滩(2.83±1.67 mg (C)/(m2 ·h))和芦苇地(3.82±2.50 mg (C)/(m2 ·h))的CH4总排放.利用扩散模型法在光滩、苔草和芦苇3个生境的CH4 Fdiff分别介于0.04~0.31、0.07~0.33和0.07~0.15 mg (C)/(m2 ·h)之间,分别占CH4总排放的3.91 %、2.55 %和1.34 %;而光滩、苔草和芦苇3个生境的Febul分别介于0.07~12.77、0.07~22.50和0.01~16.09 mg (C)/(m2 ·h)之间,分别占CH4总排放的96.09 %、97.45 %和98.66 %. CH4总排放通量在洪水初期(7月12日-8月3日)最高,且大多通过气泡排放,占CH4总排放的比例达98.86 % ~99.18 %;随着淹水时间增加(8月3日-11月4日),CH4总排放通量逐渐降低,而通过扩散排放的量逐渐增加,在淹水后期(10月17日-11月4日),扩散排放通量占CH4总排放的比例达63.47 % ~81.97 % (图 3).
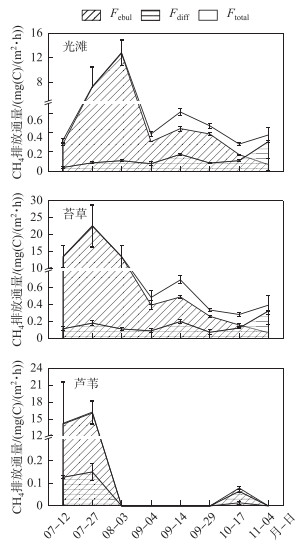
|
图 3 不同生境CH4排放通量时间动态 Fig.3 Temporal dynamic of CH4 emission fluxes in three sampling sites |
CH4总排放通量、扩散排放通量以及气泡排放通量与水体环境因子间的逐步回归分析表明:水温(T)是影响CH4总排放(P < 0.01)和气泡排放(P=0.001)的关键因子,而CH4扩散排放主要受水温、电导率(EC)和pH影响(P < 0.01;表 3).进一步线性回归分析表明:CH4总排放(R2=0.65)和气泡排放(R2=0.66)与水温呈正相关,CH4扩散排放与水温(R2=0.08)和电导率(R2 =0.38)均呈负相关,与pH (R2=0.14)呈正相关(图 4).以上结果表明水温增加促进了CH4气泡排放和CH4总排放,但抑制了CH4的扩散排放.此外,水体电导率增加也对CH4扩散排放具有显著抑制效应,而pH的增加则显著促进CH4的扩散排放.
| 表 3 CH4排放与水体环境间的逐步回归模型 Tab. 3 The stepwise regression analysis between CH4 emission and environmental factors |

|
图 4 CH4排放与关键环境因子间的关系 Fig.4 Relationships between CH4 fluxes and key environmental factors |
通过逐步回归分析得到水温对CH4总排放、气泡排放和扩散排放均有着显著性影响,因此将CH4气泡排放和CH4扩散排放对总排放的贡献度与水温做了相关性拟合分析,分析得到随着水温增加,CH4扩散通量占CH4总排放通量的百分比逐渐降低(R2=0.88),而气泡排放通量所占百分比则逐渐增加(R2=0.87).当水温为11.7℃时,气泡排放通量和扩散排放通量各占CH4总排放通量的50 % (图 5).
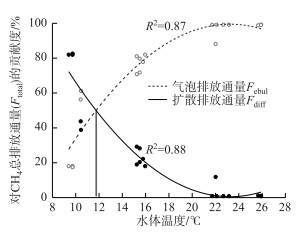
|
图 5 CH4气泡排放与扩散排放对总排放的贡献度与水温的相关关系 Fig.5 The relationship between the contribution of CH4 ebullition and diffusion emissions to total CH4 emissions and water temperature |
综合洪水期不同生境不同日期测得的CH4排放通量算出洞庭湖洪水期CH4 Ftotal均值为5.53±1.70 mg (C)/(m2 ·h),其中Fdiff均值为0.13±0.017 mg (C)/(m2 ·h),占总排放的2.63 %,而Febul均值为5.40±1.68 mg (C)/(m2 ·h),占总排放的97.37 %. 3种生境中,苔草地的CH4总排放高于光滩和芦苇地的CH4排放(图 3).湿地沉积物中产甲烷菌和甲烷氧化菌的相对丰度是影响CH4排放的重要因素[31-32],而洞庭湖湿地洪水期苔草地产甲烷菌和甲烷氧化菌的相对丰度比例最高(未发表数据),由此导致洪水期苔草地的CH4总排放最高.此外,苔草植被的传输作用也可能是苔草地CH4排放量最高的重要原因之一[33-34].
与国外其他湖泊相比(表 4),洞庭湖洪水期CH4排放处于中等水平,湖泊CH4排放通量和排放途径受微地形、植被、水文、微生物活性、可利用性有机质和CH4产生速率等多种因素的影响[9, 35-36],会随着地理位置和气候条件的改变而发生显著变化,如在加拿大北方森林浅水湖泊中,温度在5~25℃之间,CH4气泡排放通量和扩散排放通量均值分别为1.1和2.2 mg (C)/(m2 ·h),分别占总排放(3.3 mg (C)/(m2 ·h))的22 %和78 % [9];而在夏季的加利福尼亚州南部另外一个浅水Elsinore湖,白天温度最高达到35℃,CH4气泡排放的通量在13~96 mg (C)/(m2 ·h)范围内,占总排放的90 %以上[37](表 4).湖泊水温可能是引起CH4排放通量区域差异的主要原因.
| 表 4 不同湖泊的CH4气泡排放通量以及CH4扩散排放通量 Tab. 4 The CH4 ebullitive flux and CH4 diffusion flux in different lakes |
温度是影响湖泊CH4排放的重要因素[41-42].特别是在夏季,湖泊CH4排放对温度的敏感度最高[43].在本研究中,CH4总排放通量和气泡排放通量均与水温呈显著正相关,CH4扩散排放通量与水温关系相对较弱(R2=0.08).淹水初期(7月12日-8月3日)的水温在20℃以上,这时CH4总排放最高,且大多通过气泡排放,随着温度降低,CH4气泡排放通量占CH4总排放通量的百分比逐渐减少,而CH4扩散排放通量所占百分比逐渐增加.在温度大于11.7℃时,水体中CH4主要通过气泡排放,在温度小于11.7℃时,水体CH4主要通过扩散排放. DelSontro等[9]同样研究发现CH4气泡排放和扩散排放占总排放的比例受温度变化的影响,且温度对CH4气泡排放的影响要高于对CH4扩散排放的影响.而Xiao等[44]在对同一气候带浅水池塘的研究中也发现,在夏季超过90 %的CH4是通过气泡释放.这可能是因为温度升高导致产甲烷菌活性和CH4产生速率增加,同时水温升高降低了水体CH4饱和浓度,从而促进气泡的形成和释放[9, 16].此外,水温增加也会导致CH4氧化速率增加[17],使得扩散排放的CH4在到达水面前被大量氧化,减轻了CH4扩散排放.因此,水温有可能是决定该研究水体中CH4排放途径是以扩散排放为主还是气泡排放为主的决定影响因素之一.
在本研究中,水体pH值仅与CH4扩散通量呈正相关,对CH4的总排放和气泡排放影响较弱.水体pH主要通过影响产甲烷菌和甲烷氧化菌的活性来影响水体CH4浓度和扩散通量[45].产甲烷菌对水体pH值变化较为敏感,大部分产甲烷菌在pH值为6~8的环境中才能进行产CH4活动[46].而本研究3种生境类型的水样pH均值介于7.59~8.01之间,几乎都处于产甲烷菌的最适pH值活动范围内且pH变异较小.因此,水体pH对CH4扩散通量的影响相对较小(R2=0.14).
水体电导率越高,表明水体中电子受体数量越多,这些电子受体与产甲烷菌竞争电子,从而抑制了CH4的产生[47].国内外关于盐沼[48-49]和潮汐淡水湿地[45, 50-51]的研究中均发现:CH4排放通量与电导率呈显著负相关,而在本研究中,水体电导率仅与CH4扩散排放呈显著负相关(R2=0.38),与CH4总排放和气泡排放无显著相关性.这可能是因为淡水湖泊中会发生反硝化型厌氧甲烷氧化,而水体电导率升高会导致这一厌氧氧化菌所利用的电子受体数量增加,促进了CH4厌氧氧化[52].实际上,在淹水初期CH4产生浓度较高的情况下,CH4大部分通过气泡排放,从而避开了通过沉积物-界面和水体传输过程中的氧化消耗[6, 53],而通过扩散排放的CH4由于在水体滞留时间相对较长,在扩散传输过程中更容易发生厌氧氧化[54].
3.3 不确定性分析本研究结果还存在一些不确定性,芦苇地由于淹水时间短,采样次数少;同时,在芦苇地采样时避开了露在水面上的芦苇,而芦苇地CH4排放除了通过气泡和扩散排放,也有可能是通过露在水面上的芦苇传输到大气中[34],因此可能低估芦苇地CH4排放通量.另外,漂浮箱法由于对水面的扰动可能会对实际排放通量造成低估[55],模型法也可能因为对温度、风速、河水流速等多种环境因素把握不充分而使估算的扩散排放通量产生误差.但本文主要考察的是CH4在水体中的传输途径及其相对贡献,总体而言,以上不确定性对本文的结论无较大影响.
4 结论与展望 4.1 结论1) 洞庭湖湿地洪水期水体CH4排放以气泡排放为主.洞庭湖洪水期CH4 Ftotal均值为5.53±1.70 mg (C)/(m2 ·h),其中Fdiff均值为0.13±0.017 mg (C)/(m2 ·h),占总排放的2.63 %,而Febul均值为5.40±1.68 mg (C)/(m2 ·h),占总排放的97.37 %.
2) 水温是影响CH4总排放通量、CH4扩散排放通量和CH4气泡排放通量的关键因子,除此之外,CH4扩散排放还受水体电导率和pH的影响.
3) 水体CH4传输方式并非一成不变,而是随着水体温度的改变随之发生变化.在洞庭湖湿地,当水体温度较低时(低于11.7℃),水体CH4排放以扩散排放为主,但当水体温度较高时(高于11.7℃),水体CH4排放多通过气泡排放.
4.2 展望当前,全球湿地CH4排放估算还存在较大不确定性(40 %),一方面是由于对不同湿地类型研究的不均衡[56];另一方面则是对水体CH4排放过程还缺乏清晰认识[57].本研究以位于亚热带区域的洞庭湖湿地水体为研究对象,估算了水体CH4扩散排放和气泡排放在CH4总排放中的贡献,对于揭示中低纬度内陆湖泊水体CH4排放过程有重要意义.由于CH4气泡排放的速率和量级要远大于扩散排放,因此,在中低纬度内陆湖泊湿地中,未来应加强CH4的气泡排放过程及环境影响研究;其次,由于CH4的传输方式会随着水体温度的变化而发生改变,如在本研究中发现水温11.7℃是CH4扩散方式发生转变的温度阈值.因此,未来在全球CH4排放估算研究中应根据水体温度环境建立合适的CH4估算模型.
致谢: 感谢中国科学院洞庭湖湿地生态系统观测研究站以及中国科学院亚热带农业生态研究所农业生态过程重点实验室的各位老师在本研究样品采集和分析过程中给予的支持和帮助.
| [1] |
International Panel on Climate Change [IPCC]. Climate change: Synthesis report. In: Team CW, Pachauri RK, Meyer LA eds. Contribution of Working Groups Ⅰ, Ⅱ and Ⅲ to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Geneva, Switzerland, 2014: 151.
|
| [2] |
Bloom AA, Palmer PI, Fraser A et al. Large-scale controls of methanogenesis inferred from methane and gravity spaceborne data. Science, 2010, 327(5963): 322-325. DOI:10.1126/science.1175176 |
| [3] |
Winton RS, Flanagan N, Richardson CJ. Neotropical peatland methane emissions along a vegetation and biogeochemical gradient. PLoS One, 2017, 12(10): e0187019. DOI:10.1371/journal.pone.0187019 |
| [4] |
Lin M, Xu M, Geng YQ et al. Spatial heterogeneity and controlling factors of sutumn CH4 flux at water -air interface in Poyang lake of Jiangxi Province, China. Chinese Journal of Ecology, 2012, 31(8): 2112-2118. [林茂, 徐明, 耿玉清等. 鄱阳湖秋季水-气界面CH4排放通量的区域差异及影响因素. 生态学杂志, 2012, 31(8): 2112-2118. DOI:10.13292/j.1000-4890.2012.0257] |
| [5] |
Glaser PH, Chanton JP, Morin P et al. Surface deformations as indicators of deep ebullition fluxes in a large northern peatland. Global Biogeochemical Cycles, 2004, 18(1). DOI:10.1029/2003GB002069 |
| [6] |
Horn C, Metzler P, Ullrich K et al. Methane storage and ebullition in monimolimnetic waters of polluted mine pit lake Vollert-Sued, Germany. Science of the Total Environment, 2017, 584/585: 1-10. DOI:10.1016/j.scitotenv.2017.01.151 |
| [7] |
Bastviken D, Cole JJ, Pace ML et al. Measurement of methane oxidation in lakes: a comparison of methods. Environmental Science & Technology, 2002, 36(15): 3354-3361. DOI:10.1021/es010311p |
| [8] |
Mcginnis DF, Kirillin G, Tang KW et al. Enhancing surface methane fluxes from an oligotrophic lake: exploring the microbubble hypothesis. Environmental Science & Technology, 2015, 49(2): 873-880. DOI:10.1021/es503385d |
| [9] |
Delsontro T, Boutet L, St-Pierre A et al. Methane ebullition and diffusion from northern ponds and lakes regulated by the interaction between temperature and system productivity. Limnology and Oceanography, 2016, 61(S1): S62-S77. DOI:10.1002/Ino.10335 |
| [10] |
Casper P, Maberly SC, Finlay HBJ. Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry, 2000, 49(1): 1-19. DOI:10.2307/1469408 |
| [11] |
Repo ME, Huttunen JT, Naumov AV et al. Release of CO2 and CH4 from small wetland lakes in western Siberia. Tellus Series B- Chemical and Physical Meteorology, 2007, 59(5): 788-796. DOI:10.1111/j.1600-0889.2007.00301.x |
| [12] |
Zhu R, Liu Y, Xu H et al. Carbon dioxide and methane fluxes in the littoral zones of two lakes, east Antarctica. Atmospheric Environment, 2010, 44(3): 304-311. DOI:10.1016/j.atmosenv.2009.10.038 |
| [13] |
Biderre-Petit C, Didier J, Dugat-Bony E et al. Identification of microbial communities involved in the methane cycle of a freshwater meromictic lake. FEMS Microbiology Ecology, 2011, 77(3): 533-545. DOI:10.1111/j.1574-6941.2011.01134.x |
| [14] |
Yan XC, Zhang CQ, Ji M et al. Concentration of dissolved greenhouse gas and its influence factors in the summer surface water of eutrophic lake. J Lake Sci, 2018, 30(5): 1420-1428. [闫兴成, 张重乾, 季铭等. 富营养化湖泊夏季表层水体温室气体浓度及其影响因素. 湖泊科学, 2018, 30(5): 1420-1428. DOI:10.18307/2018.0523] |
| [15] |
Delsontro T, Mcginnis DF, Sobek S et al. Extreme methane emissions from a swiss hydropower reservoir: contribution from bubbling sediments. Environmental Science & Technology, 2010, 44(7): 2419-2425. DOI:10.1021/es9031369 |
| [16] |
Borrel G, Jézéquel D, Biderre-Petit C et al. Production and consumption of methane in freshwater lake ecosystems. Research in Microbiology, 2011, 162(9): 832-847. DOI:10.1016/j.resmic.2011.06.004 |
| [17] |
Semrau JD, DiSpirito AA, Yoon S. Methanotrophs and copper. FEMS Microbiology Reviews, 2010, 34(4): 496-531. DOI:10.1111/j.1574-6976.2010.00212.x |
| [18] |
Huang Q, Sun ZD, Jiang JH. Impacts of the operation of the Three Gorges Reservoir on the lake water level of Lake Dongting. J Lake Sci, 2011, 23(3): 424-428. [黄群, 孙占东, 姜加虎. 三峡水库运行对洞庭湖水位影响分析. 湖泊科学, 2011, 23(3): 424-428. DOI:10.18307/2011.0316] |
| [19] |
Li F, Hou ZY, Chen XS et al. Study on composition and floristic of vegetation in Dongting Lake. Research of Agricultural Modernization, 2016, 31(3): 347-351. [李峰, 侯志勇, 陈心胜等. 洞庭湖湿地植被组成及区系成分分析. 农业现代化研究, 2016, 31(3): 347-351.] |
| [20] |
Deng Z, Li Y, Xie Y et al. Hydrologic and edaphic controls on soil carbon emission in Dongting Lake Floodplain, China. Journal of Geophysical Research-Biogeosciences, 2018, 123(9): 3088-3097. DOI:10.1029/2018JG004515 |
| [21] |
Zheng XH, Wang R. Terrestrial ecosystems-atmosphere: measurement methods for exchange flux of carbon and nitrogen gases. Bejing: China Meteorological Press, 2017: 58-65. [郑循华, 王睿. 陆地生态系统-大气:碳氮气体交换通量的地面观测方法. 北京: 气象出版社, 2017: 58-65.]
|
| [22] |
Wanninkhof R. Relationship between wind speed and gas exchange over the ocean. Journal of Geophysical Research-Oceans, 1992, 97(C5): 7373-7382. DOI:10.1029/92JC00188 |
| [23] |
Yan WJ, Wang B, Li XY et al. Summary of studies on environmental chemical process of dissolved N2O in rivers and the exchange flux between water-air interface. Journal of Agro-Environment Science, 2008, 27(1): 15-22. [晏维金, 王蓓, 李新艳等. 河流溶存N2O的环境化学过程及其在水—气界面交换过程的研究. 农业环境科学学报, 2008, 27(1): 15-22. DOI:10.3321/j.issn:1672-2043.2008.01.002] |
| [24] |
Liss PS, Merlivat L. Air-sea gas exchange rates: Introduction and synthesis. Netherlands: Springer, 1986: 185 : 113-127. DOI: 10.1007/978-94-009-4738-2_5.
|
| [25] |
Raymond PA, Cole JJ. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries, 2001, 24(2): 312-317. DOI:10.2307/1352954 |
| [26] |
Amouroux D, Roberts G, Rapsomanikis S et al. Biogenic gas (CH4, N2O, DMS) emission to the atmosphere from near-shore and shelf waters of the north-western black sea. Estuarine Coastal and Shelf Science, 2002, 54(3): 575-587. DOI:10.1006/ecss.2000.0666 |
| [27] |
Zappa CJ. Microbreaking and the enhancement of air-water transfer velocity. Journal of Geophysical Research, 2004, 109(C8): C08S16. DOI:10.1029/2003jc001897 |
| [28] |
Wang Q, Guan DM, Li MH et al. Distribution and atmospheric fluxes of CO2, CH4 and N2O in surface water of Dalian Bay. Marine Environmental Science, 2011, 30(3): 398-403. [王芹, 关道明, 李明浩等. 大连湾表层海水CO2、CH4和N2O的分布及海-气交换通量. 海洋环境科学, 2011, 30(3): 398-403. DOI:10.3969/j.issn.1007-6336.2011.03.021] |
| [29] |
Gao J, Zheng XH, Wang R et al. Preliminary comparisons of the static floating chamber and the diffusion model methods for measuring water-Atmosphere exchanges of methane and nitrous oxide from inland water bodies. Climatic and Environmental Research, 2014, 19(3): 290-302. [高洁, 郑循华, 王睿等. 漂浮通量箱法和扩散模型法测定内陆水体CH4和N2O排放通量的初步比较研究. 气候与环境研究, 2014, 19(3): 290-302.] |
| [30] |
Casper P, Albino MF, Adams DD. Diffusive fluxes of CH4 and CO2 across the water-air interface in the eutrophic Lake Dagow, northeast Germany. SIL Proceedings (1922-2010), 2009, 30(6): 874-877. DOI:10.1080/03680770.2009.11902261 |
| [31] |
Steger K, Premke K, Gudasz C et al. Comparative study on bacterial carbon sources in lake sediments: the role of methanotrophy. Aquatic Microbial Ecology, 2015, 76(1): 39-47. DOI:10.3354/ame01766 |
| [32] |
Chen X, Ma H, Zheng Y et al. Changes in methane emission and methanogenic and methanotrophic communities in restored wetland with introduction of Alnus trabeculosa. Journal of Soils & Sediments, 2016, 17(1): 1-9. DOI:10.1007/s11368-016-1496-0 |
| [33] |
Kankaala P, Tiina K, Suvi M et al. Methane efflux in relation to plant biomass and sediment characteristics in stands of three common emergent macrophytes in boreal mesoeutrophic lakes. Global Change Biology, 2010, 11(1): 145-153. DOI:10.1111/j.1365-2486.2004.00888.x |
| [34] |
Sun X, Song C, Guo Y et al. Effect of plants on methane emissions from a temperate marsh in different seasons. Atmospheric Environment, 2012, 60: 277-282. DOI:10.1016/j.atmosenv.2012.06.051 |
| [35] |
Wik M, Crill PM, Varner RK et al. Multiyear measurements of ebullitive methane flux from three subarctic lakes. Journal of Geophysical Research-Biogeosciences, 2013, 118(3): 1307-1321. DOI:10.1002/jgrg.20103 |
| [36] |
Riutta T, Laine J, Aurela M et al. Spatial variation in plant community functions regulates carbon gas dynamics in a boreal fen ecosystem. Tellus Series B- Chemical and Physical Meteorology, 2007, 59(5): 838-852. DOI:10.1111/j.1600-0889.2007.00302.x |
| [37] |
Martinez D, Anderson MA. Methane production and ebullition in a shallow, artificially aerated, eutrophic temperate lake (Lake Elsinore, CA). Science of the Total Environment, 2013, 454: 457-465. DOI:10.1016/j.scitotenv.2013.03.040 |
| [38] |
Walter KM, Zimov SA, Chanton JP et al. Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature, 2006, 443(7107): 71-75. DOI:10.1038/nature05040 |
| [39] |
Rinta P, Bastviken D, Schilder J et al. Higher late summer methane emission from central than northern European lakes. Journal of Limnology, 2017, 76(1): 52-67. DOI:10.4081/jlimnol.2016.1475 |
| [40] |
Michael K. Methane emission by bubbling from Gatun Lake, Panama. Journal of Geophysical Research-Atmospheres, 1994, 99(D4): 8307-8319. DOI:10.1029/92JD02170 |
| [41] |
Palma-Silva C, Marinho CC, Albertoni EF et al. Methane emissions in two small shallow neotropical lakes: The role of temperature and trophic level. Atmospheric Environment, 2013, 81(12): 373-379. DOI:10.1016/j.atmosenv.2013.09.029 |
| [42] |
Rõõm EI, Nõges P, Feldmann T et al. Years are not brothers: two-year comparison of greenhouse gas fluxes in large shallow Lake Võrtsjärv, Estonia. Journal of Hydrology, 2014, 519: 1594-1606. DOI:10.1016/j.jhydrol.2014.09.011 |
| [43] |
Liu L, Xu M, Li R et al. Timescale dependence of environmental controls on methane efflux from Poyang Hu, China. Biogeosciences, 2017, 14(8): 2019-2032. DOI:10.5194/bg-14-2019-2017 |
| [44] |
Xiao S, Yang H, Liu D et al. Gas transfer velocities of methane and carbon dioxide in a subtropical shallow pond. Tellus Series B-chemical and Physical Meteorology, 2014, 66(1): 23795. DOI:10.3402/tellusb.v66.23795 |
| [45] |
Chen H, Zhou S, Wu N et al. Advance in studies on production, oxidation and emission flux of methane from wetlands. Chinese Journal of Applied and Environmental Biology, 2006, 12(5): 726-733. [陈槐, 周舜, 吴宁等. 湿地甲烷的产生、氧化及排放通量研究进展. 应用与环境生物学报, 2006, 12(5): 726-733. DOI:10.3321/j.issn:1006-687X.2006.05.029] |
| [46] |
Yang SS, Chen IC, Ching-Pao L et al. Carbon dioxide and methane emissions from Tanswei River in northern Taiwan. Atmospheric Pollution Research, 2015, 6(1): 52-61. DOI:10.5094/APR.2015.007 |
| [47] |
Schlesinger, William H, Bernhardt ES. Biogeochemistry: an analysis of global change. Quarterly Review of Biology, 1997, 54(4): 353-423. DOI:10.1016/C2012-0-01654-7 |
| [48] |
Zhao J, Zhang GL, Wu Y et al. Distribution and emission of methane from the Changjiang. Environmental Science, 2011, 32(1): 18-25. [赵静, 张桂玲, 吴莹等. 长江中溶存甲烷的分布与释放. 环境科学, 2011, 32(1): 18-25. DOI:10.13227/j.hjkx.2011.01.003] |
| [49] |
Sun Z, Wang L, Tian H et al. Fluxes of nitrous oxide and methane in different coastal Suaeda salsa marshes of the Yellow River estuary, China. Chemosphere, 2013, 90(2): 856-865. DOI:10.1016/j.chemosphere.2012.10.004 |
| [50] |
Wan SA, Xu H, Tong C. Monthly variations of methane emission and soil methane production potential of tidal marshes in the Min River Estuary. Wetland Science, 2015, 13(4): 417-423. [万斯昂, 徐辉, 仝川. 闽江河口潮汐沼泽甲烷排放通量和土壤甲烷产生潜力的月动态. 湿地科学, 2015, 13(4): 417-423.] |
| [51] |
Zhang JY, Zhang YY, Tong C et al. Short-term effects of saltwater intrusion and organic carbon loading on CH4 and N2O flux from estuarine freshwater marsh ecosystem. Journal of Subtropical Resources and Environment, 2016, 11(2): 39-47. [张璟钰, 章吟遥, 仝川等. 盐水入侵及有机碳输入对河口淡水沼泽CH4、N2O通量影响的短时效应. 亚热带资源与环境学报, 2016, 11(2): 39-47. DOI:10.3969/j.issn.1673-7105.2016.02.006] |
| [52] |
Tang Q, Xue XF, Wang H et al. New knowledge of methanogens and methanotrophs in lake ecosystems. J Lake Sci, 2018, 30(3): 597-610. [唐千, 薛校风, 王惠等. 湖泊生态系统产甲烷与甲烷氧化微生物研究进展. 湖泊科学, 2018, 30(3): 597-610. DOI:10.18307/2018.0302] |
| [53] |
Clough TJ, Bertram JE, Sherlock RR et al. Comparison of measured and EF5-r-derived N2O fluxes from a spring-fed river. Global Change Biology, 2006, 12(2): 352-363. DOI:10.1111/j.1365-2486.2005.01092.x |
| [54] |
Bastviken D, Cole JJ, Pace ML et al. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. Journal of Geophysical Research-Biogeosciences, 2008, 113(G2): 61-74. DOI:10.1029/2007JG000608 |
| [55] |
Broecker W, Mix A, Aneree M et al. Radiocarbon measurements on coexisting benthic and planktic foraminifera shells: Potential for reconstructing ocean ventilation times over the past 20000 years. Nuclear Instruments and Methods in Physics Research Section B- Beam Interactions with Materials and Atoms, 1984, 5(2): 331-339. DOI:10.1016/0168-583X(84)90538-X |
| [56] |
Wik M, Varner RK, Anthony KW et al. Climate-sensitive northern lakes and ponds are critical components of methane release. Nature Geoscience, 2016, 9(2): 99-105. DOI:10.1038/NGEO2578 |
| [57] |
Iwata H, Hirata R, Takahashi Y et al. Partitioning eddy-covariance methane fluxes from a shallow lake into diffusive and ebullitive fluxes. Boundary-Layer Meteorology, 2018, 169(3): 413-428. DOI:10.1007/s10546-018-0383-1 |
 2019, Vol. 31
2019, Vol. 31 


