随着经济社会发展,氮(N)、磷(P)等营养物质大量流入水体而引起湖泊富营养化.目前,该问题十分严峻,已成为世界广泛关注的环境问题之一.在富营养化水体中,蓝藻大量繁殖后其它藻类和水生植物大量减少,生物多样性明显降低[1].
蓝藻通过次生代谢途径产生大量的挥发性有机化合物(volatile organic compounds, VOCs),根据其类型可分为萜烯类、醛类、醇类、酯类、酮类、烃类、苯类和硫化物[2-5].铜绿微囊藻(Microcystis aeruginosa)主要释放C15~C20脂肪族烃、萜烯类、臭樟脑、β-环柠檬醛和β-紫罗酮等化合物[2],同时在NaNO3胁迫下其VOCs种类与释放量均明显增加[3]. Fujise等[4]研究发现,颤藻(Oscillatoria)、微囊藻和鱼腥藻(Anabeana)3种蓝藻均会释放大量的2-甲基异莰醇和土味素,从而导致水体呈现泥土气味.我们在早期研究中发现,无氮条件可促进水华微囊藻(M. flos-aquae) VOCs释放,同时这些VOCs可抑制小球藻(Chlorella vulgaris)细胞生长[5].对莱茵衣藻(Chlamydomonas reinhardtii)进行乙酸、NaCl和Na2CO3胁迫后,其释放的VOCs可为其他莱茵衣藻细胞传递胁迫信息[6-7]. Fink等[8]研究发现,丝藻(Ulothrix fimbriata)所释放的VOCs可吸引卵萝卜螺(Radix ovata)进行取食,其中3种脂氧合酶化合物和2(E), 4(E)-庚二烯醛是发挥作用的主要功能成分.由此可见,藻类所释放的VOCs可作为藻类之间以及藻类与其它生物间的信号物质之一,从而在水域生态系统中发挥信息传递的作用.
当铜绿微囊藻在培养基中培养35 d,氮源营养被完全耗尽时,其醇类VOCs(2-甲基-1-丁醇、3-甲基-1-丁醇和2-苯乙醇)和β-环柠檬醛释放量明显增加[9].分别采用NaNO3、NaNO2、NH4Cl、尿素、丝氨酸、赖氨酸和精氨酸作为氮源培养水华微囊藻,其释放的VOCs存在明显差异,并且VOCs释放量和种类均随氮量减少而增加[5].由此可见,水体中的营养状况可明显影响蓝藻次生代谢,从而改变其VOCs释放.
在富营养化水体中,P元素易形成不溶性盐而发生沉降,因此被普遍认为是藻类生长的限制性元素[10-12].此外,蓝藻在大量繁殖过程中还会消耗大量P营养,从而进一步导致P营养缺乏. Fox河水含磷量约为828 nmol/L,而其汇入的密歇根湖绿湾中的P含量由于生物固定和自然沉降则降低了40倍[13]. Steinhart等[14]研究智利南部28个湖泊表明,半数的湖泊呈现缺P状态.在兴凯湖和唐岛湾养殖区,由于浮游生物的影响,均呈现出季节性缺P现象[15-16].许海等[17]通过对太湖监测表明,在水华暴发期间P浓度很低;同时其室内研究发现,铜绿微囊藻对P饥饿具有很强的耐受力,可维持8 d的指数生长.然而,在缺P条件下,蓝藻细胞释放的VOCs会发生何种变化,同时这些VOCs对蓝藻和其他藻类具有何种影响?目前尚不清楚.当蓝藻大量繁衍时,绿藻、硅藻等藻类大量减少[1].衣藻隶属于绿藻门,广泛存在于河流、湖泊、海洋等水体中.因此,本研究通过分析蓝藻水华的主要种类铜绿微囊藻在无P条件下产生的VOCs以及VOCs对莱茵衣藻的影响,以期从理论上探索P营养缺乏诱导蓝藻释放VOCs的过程,并揭示VOCs在蓝藻低P胁迫下维持种群竞争优势和藻类群落结构多样性减少中的作用.
1 材料与方法 1.1 材料铜绿微囊藻FACHB-1028株系购自中国科学院水生生物研究所淡水藻种库,隶属于蓝藻门(Cyanophyta)微囊藻科(Microcystaceae)微囊藻属(Microcystis).莱茵衣藻CC1690 (中国科学院遗传与发育生物学研究所刘翠敏研究员惠赠)隶属于绿藻门(Chlorophyta)衣藻科(Chlamydomonadaceae)衣藻属(Chlamydomonas).分别采用BG-11和TAP培养基培养铜绿微囊藻和莱茵衣藻,培养条件为光照(16 h)/黑暗(8 h)、光强50 μmol/(m2·s)、温度25℃.
1.2 VOCs处理莱茵衣藻将铜绿微囊藻离心后分别转入300 ml BG-11无P和标准培养基中,藻细胞密度为1×107 cells/ml.根据文献[6],将VOCs(流速0.01 m3/min)通入300 ml莱茵衣藻培养液中,其藻细胞密度为4×106 cells/L.以通入无藻培养基中的空气作为对照,3次重复. 24 h后,测定莱茵衣藻的细胞密度、光合色素含量和叶绿素荧光特性.
1.3 实验方法 1.3.1 细胞密度测定采用血球计数板(25×16型)计数藻细胞密度,根据公式(1)进行计算.每次计数6次,采用其平均值表示藻细胞密度(cells/ml).
| $ 藻细胞密度 = 实测数/80 \times 400 \times 10000 \times 稀释倍数 $ | (1) |
根据甘丽平等[3]的方法,利用QC-2型大气采样仪(北京市劳动保护科学研究所科技发展公司)通过动态顶空套袋法收集在BG-11标准培养基和无P培养基中培养24 h的铜绿微囊藻所释放的VOCs,藻细胞密度为5×106 cells/L,采气流量0.3 m3/min,采气时间40 min.对室内空气进行采气,作为空白对照.利用热脱附/气相色谱/质谱联用法(TDS/GC/MS)分析气体组分,TDS(TDS-3型, 德国Gerstel公司)系统载气压力20 kPa,进样口温度250℃,脱附温度250℃保持10 min,冷阱温度-120℃保持3 min,冷阱进样时温度降至260℃. GC (GC 7890A, 美国Agilent公司)色谱柱为HP-5MS柱(30 m×250 μm×0.25 μm),测定时初始温度为40℃,保持4 min后以6℃/min的速率升至250℃,保持3 min后以10℃/min的速率升温到270℃并保持5 min. MS (MS 5975C, 美国Agilent公司)采用EI方式进行电离,电子能量70 eV,质量范围28~450 aum,接口温度280℃,离子源温度230℃,四级杆温度150℃.
通过检索NIST/EPA/NIH质谱库(NIST 08)并结合文献[2, 6-7]报道的VOCs保留时间确定VOCs成分并获得GC/MS质量数据. VOCs收集完成后测定藻细胞密度,并以107个细胞释放的VOCs峰面积表示VOCs成分的相对含量.室内空气空白对照中VOCs成分很少、含量很低,对藻类VOCs分析无影响,同时空气中的VOCs含量在藻类VOCs计算时进行扣除.
1.3.3 光合色素含量测定3 ml莱茵衣藻培养液离心后,重悬于3 ml 80 %丙酮中并置于4℃冰箱中静置提取24 h.待光合色素提取完成后,采用Lichtenthaler等[18]的方法测定提取液中叶绿素a、叶绿素b和类胡萝卜素含量(μg/106 cells).
| $ {C_{\rm{a}}} = 12.21\;{\rm{O}}{{\rm{D}}_{663}} - 2.81\;{\rm{O}}{{\rm{D}}_{646}} $ | (2) |
| $ {C_{\rm{b}}} = 20.13\;{\rm{O}}{{\rm{D}}_{646}} - 5.03\;{\rm{O}}{{\rm{D}}_{663}} $ | (3) |
| $ {C_{{\rm{x}} \cdot {\rm{c}}}} = (1000\;{\rm{O}}{{\rm{D}}_{470}} - 3.27{C_{\rm{a}}} - 104\;{C_{\rm{b}}})/229 $ | (4) |
式中, Ca、Cb分别为叶绿素a和b含量;Cx ·c为类胡萝卜素含量.
1.3.4 叶绿素荧光测定取一定量莱茵衣藻细胞培养液(约1×107 cells)离心后,重悬于10 μl培养基中并滴于3 cm2滤纸上以形成约0.5 cm2斑点[19].黑暗中放置15 min后,采用PAM-2500 (Walz, 德国)测定其光系统Ⅱ(PSⅡ)最大光化学量子产量(Fv/Fm)、有效光化学量子产量[Y(Ⅱ)]、光化学淬灭系数(qP)、非光化学淬灭系数(NPQ)以及光合电子传递速率(ETR)[20].
1.4 数据处理采用响应指数(response index, RI)[21]表示铜绿微囊藻所释放的VOCs对莱茵衣藻细胞生长、光合色素含量和光合性能的影响:
| $ RI = \left\{ \begin{array}{l} 1 - {\rm{C}}/{\rm{T}}\left( {当{\rm{T}} \ge {\rm{C}}} \right)\\ {\rm{T}}/{\rm{C}} - 1\left( {当{\rm{T < C}}} \right) \end{array} \right. $ | (5) |
式中, T为铜绿微囊藻VOCs通入莱茵衣藻培养液(处理),C为无藻培养基中的空气通入莱茵衣藻培养液(对照),当RI>0时表明铜绿微囊藻VOCs对莱茵衣藻具有促进作用,当RI < 0时则表明具有抑制作用.
实验中所测定的原始数据采用SPSS 12.0软件通过t检验进行方差分析,计算出RI后利用Origin 8.0软件进行作图.
2 结果 2.1 无P条件对铜绿微囊藻VOCs释放的影响铜绿微囊藻在BG-11标准培养基中培养24 h后,其释放的VOCs中含有26种化合物.根据化合物性质可分为硫化物、烃类、萜烯类、苯类、醛类、酮类和酯类,其中甲基异丙基二硫醚(7.13×106/107 cells)、异丙基(化)二硫(2.26×106/107 cells)、桉树脑(3.11×106/107 cells)、长叶烯(2.60×106/107 cells)、十五烷(2.22×106/107 cells)和丙酸-2-甲基-1-(1, 1-二甲基乙基)-2-甲基-1, 3-丙二基酯(5.55×106/107 cells)峰面积很大, 为其主要成分(表 1).
| 表 1 无P培养基对铜绿微囊藻VOCs释放的影响(标准培养基中所释放的VOCs引自甘丽平等[3]) Tab.1 Effects of non-P medium on VOCs emission from M. aeruginosa |
在无P培养条件下,铜绿微囊藻VOCs释放量明显增加,与标准培养基相比总释放量增加了73.4 %.此外,无P培养还诱导7种新化合物释放,分别为α-蒎烯、2-乙基己醛、1-苯基-1-丁酮、9-氧-双环[3.3.1]壬烷-2, 6-二酮、乙酸异辛酯、1H-1-亚乙基-茚和2, 6, 10-三甲基十四烷;同时(Z)-3-十二烯、(1S)-2, 2-二甲基-3-亚甲基-二环[2.2.1]庚烷、p-伞花烃、2, 6-二叔丁基对苯醌和(Z)-7-十六烯醛5种化合物消失(表 1).
2.2 铜绿微囊藻释放的VOCs对莱茵衣藻细胞生长的影响虽然铜绿微囊藻在标准培养基中所产生的VOCs对莱茵衣藻生长具有抑制作用,但是与对照相比(空气处理)并没有显著差异;而当采用无P培养基培养铜绿微囊藻后,其VOCs可明显抑制莱茵衣藻生长,RI为-0.21,并且差异达到极显著水平(P < 0.01)(图 1).

|
图 1 铜绿微囊藻释放的VOCs对莱茵衣藻细胞生长的影响 (**表示与对照相比,在P < 0.01水平上差异显著) Fig.1 Effects of M. aeruginosa VOCs on C. reinhardtii cell growth (**:Significant difference at P < 0.01 level compared to the control) |
分别采用铜绿微囊藻在标准培养基和无P培养条件下释放的VOCs处理莱茵衣藻,其叶绿素a RI分别为-0.05和-0.25(P < 0.01) (图 2A).与叶绿素a相似,无P条件下铜绿微囊藻释放的VOCs可极显著(P < 0.01)降低叶绿素b和类胡萝卜素含量,其RI分别为-0.26和-0.24(图 2B、C).
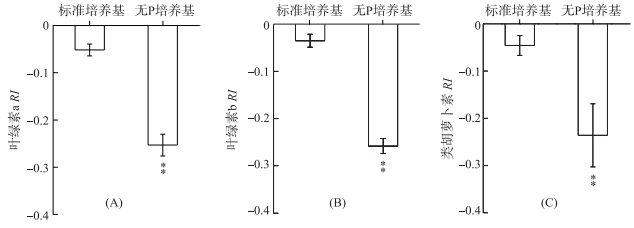
|
图 2 铜绿微囊藻释放的VOCs对莱茵衣藻光合色素的影响 (**表示与对照相比,在P < 0.01水平上差异显著) Fig.2 Effects of M. aeruginosa VOCs on photosynthetic pigments in C. reinhardtii cells (**:Significant difference at P < 0.01 level compared to the control) |
铜绿微囊藻在标准培养基中释放的VOCs对莱茵衣藻Fv/Fm无显著影响,而无P条件下释放的VOCs则能明显降低Fv/Fm,其RI为-0.08(P < 0.05) (图 3A).与Fv/Fm相似,Y(Ⅱ)、qP和ETR在无P条件下释放的VOCs处理后也均明显降低,其RI分别为-0.12、-0.16和-0.16,并且差异均达到极显著水平(P < 0.01) (图 3B、C、E).对NPQ而言,铜绿微囊藻在标准培养基和无P培养条件下释放的VOCs均使其明显升高,RI分别为0.04和0.26(P < 0.01) (图 3D).
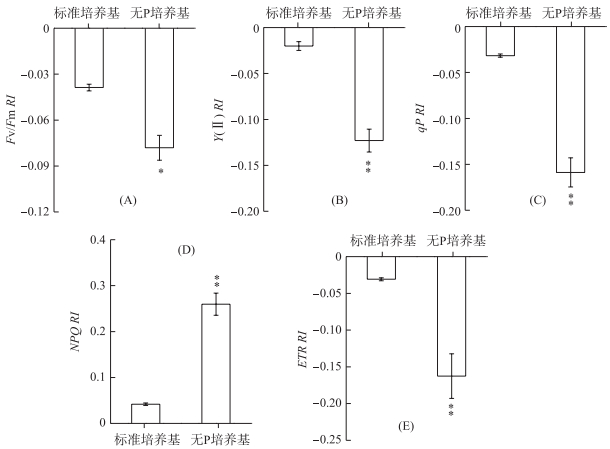
|
图 3 铜绿微囊藻释放的VOCs对莱茵衣藻光合能力的影响 (*表示与对照相比,在P < 0.05水平上差异显著; **表示与对照相比,在P < 0.01水平上差异显著) Fig.3 Effects of M. aeruginosa VOCs on the photosynthetic abilities in C. reinhardtii cell (*:Significant difference at P < 0.05 level compared to the control; **:Significant difference at P < 0.01 level compared to the control) |
藻类通过次生代谢途径合成VOCs,此过程可受到光照、温度、营养条件等环境因子的影响[9, 22-23].当减少培养基中氮营养时,水华微囊藻VOCs的种类和释放量均明显增加[5];当N营养耗尽时,铜绿微囊藻增加了醇类VOCs和β-环柠檬醛的释放量[9]. Dzialowski等[24]通过室外调查发现,水库中土味素含量与无机磷含量呈负相关.当铜绿微囊藻在无P条件下培养24 h后,其VOCs释放量明显增加,同时出现7种新化合物(表 1).由此可见,营养缺乏会促进蓝藻细胞进行次生代谢,从而释放出大量的VOCs.
植物释放的萜烯类VOCs主要有异戊二烯、单萜、倍半萜和双萜,其中倍半萜主要通过细胞质中的甲羟戊酸途径合成;而异戊二烯、单萜和双萜则主要通过质体中的甲基赤藓醇-4-磷酸途径(脱氧木酮糖-5-磷酸途径)合成[25],非生物胁迫可诱导两条途径中相关酶基因大量表达从而促进萜烯类VOCs的合成与释放[26-28].含有苯环的芳香族化合物来源于苯丙氨酸[29],例如水杨酸为含苯VOCs,病原菌侵染和O3胁迫可诱导其合成途径中相关酶基因表达从而促进其释放[30].在拟南芥(Arabidopsis thaliana)中,P缺乏可诱导1000多种基因表达,其中大量的基因与萜烯类VOCs和苯丙氨酸代谢相关[31].含硫类VOCs在植物中并不多见,可作为某些植物的特有成分,例如芥子油甙为十字花科植物所特有,其合成与释放可被昆虫取食所诱导[32].烃类和醛类属于脂肪族类化合物,其来源主要是脂肪酸脱羧反应[33].藻细胞膜脂过氧化反应或在脂酰水解酶作用下可产生游离脂肪酸,其在脂酰-脂酰载体蛋白还原酶(acyl-ACP还原酶)催化下形成脂肪醛,再经醛脱羰酶作用形成脂肪族烃[34-36].在无P条件下,铜绿微囊藻萜烯类、苯类、含硫类和烃类VOCs释放量均明显增加,这可能是无P条件诱导了相关合成酶基因表达,从而促进了这些VOCs的合成与释放,然而其具体的诱导机制尚待深入研究.
在无P条件下,2种脂肪族VOCs (Z)-3-十二烯与(Z)-7-十六烯醛、1种萜烯类VOCs (1S)-2, 2-二甲基-3-亚甲基-二环[2.2.1]庚烷和2种苯类VOCs p-伞花烃与2, 6-二叔丁基对苯醌均未被检测到,其原因可能为无P条件影响了此5种化合物相关合成酶活性,同时原底物被用于形成其它化合物.例如,无P条件下p-伞花烃的前体物质可能被用于形成o-伞花烃,从而致使o-伞花烃释放量增加,p-伞花烃消失(表 1).
在陆地生态系统中,植物释放的VOCs可作为信号物质在植物间进行信息传递[37-38],从而提高自身竞争能力并促进种群扩大繁衍[38-39].在水域生态系统中,藻类VOCs也具有信息传递的作用.例如,乙酸、NaCl和Na2CO3胁迫诱导莱茵衣藻释放的VOCs可为其他莱茵衣藻细胞传递胁迫信息,从而使其做好防御准备[6-7].采用蓝藻所释放的VOCs处理蛋白核小球藻(Chlorella pyrenoidosa)后,其细胞生长明显受抑[40].鲜啟鸣等[41]研究发现,两种水生植物金鱼藻(Ceratophyllum demersum)和苦草(Vallisneria spiralis)释放的VOCs可抑制铜绿微囊藻细胞生长.本研究表明,铜绿微囊藻在无P条件下释放的VOCs可显著抑制莱茵衣藻生长(图 1),这与N饥饿条件下水华微囊藻所释放的VOCs可抑制小球藻生长的作用[5]相似.由此推测,微囊藻在营养缺乏条件下产生的VOCs可能具有抑制其他藻细胞生长的化感作用.
在高等植物和藻类中,光合作用是最主要的物质和能量代谢途径,光合色素在此过程中具有捕获、传递和转化光能的作用.铜绿微囊藻在无P条件下释放的VOCs可明显降低莱茵衣藻中叶绿素和类胡萝卜素含量(图 2),同时其Fv/Fm、Y(Ⅱ)、qP和ETR也明显降低,而NPQ则明显升高(图 3).这表明在P营养缺乏条件下,铜绿微囊藻释放的VOCs通过降低其它藻细胞光能吸收与转化、PSⅡ电子产生与传递,并促进吸收的光能以热的形式进行耗散[42],从而降低其光合能力,进而减少光合产物积累以抑制藻细胞生长.
萜烯类VOCs在陆地生态系统中具有信息传递和化感抑制作用[38, 43].桉树脑和柠檬烯是萜烯类化合物,可抑制小球藻和莱茵衣藻的光合作用与细胞生长[44-45].在无P条件下,这2种化合物的释放量均明显增加,可能是铜绿微囊藻VOCs中发挥化感作用的主要物质成分. β-环柠檬醛是藻类VOCs的主要成分,可抑制蛋白核小球藻生长[40],并损伤谷皮菱形藻(Nitzschia palea)细胞[46].十七烷是长链烷烃,主要来自脂肪酸脱羧[47],其在水域生态系统中具有化感抑制作用[48].此外这2种物质均存在于铜绿微囊VOCs中,可能也是发挥化感作用的功能成分. Gómez-Tenorio等[49]研究表明,二甲基二硫醚具有抑制尖孢镰刀菌(Fusarium oxysporum)细胞生长和莴苣(Lactuca sativa)种子萌发的作用.铜绿微囊藻释放的VOCs中含有2种二硫醚,并且在限制P营养后释放量均明显增加,亦可能在莱茵衣藻生长中发挥化感抑制作用.此外,铜绿微囊藻还释放其它的VOCs成分,这些化合物在无P条件下释放量均明显增加,其是否也具有抑制藻细胞生长的作用,尚需单体验证实验予以揭示.
在富营养化水体中,蓝藻大量繁殖和P自身的沉降特性会导致P营养大量减少、甚至缺乏.在P营养缺乏时,蓝藻会释放出大量的VOCs,这些VOCs对其他藻细胞生长具有化感抑制作用,从而有利于蓝藻保持营养竞争优势,并进一步引起水体中藻类群落结构多样性的降低.
| [1] |
Zhang J. Response of freshwater algae to water quality in Qinshan Lake within Taihu Watershed, China. Physics and Chemistry of the Earth, Parts A/B/C, 2011, 36: 360-365. DOI:10.1016/j.pce.2010.04.018 |
| [2] |
Walsh K, Jones GJ, Dunstan RH. Effect of high irradiance and iron on volatile odour compounds in the cyanobacterium Microcystis aeruginosa. Phytochemistry, 1998, 49(5): 1227-1239. DOI:10.1016/S0031-9422(97)00943-6 |
| [3] |
Gan LP, Xu QH, Yang WT et al. The effects of NaNO3 stress on the release of VOCs from two Microcystis. Acta Hydrobiologica Sinica, 2015, 39(4): 782-788. [甘丽平, 徐庆欢, 杨王庭等. NaNO3胁迫对两种微囊藻VOCs释放的影响. 水生生物学报, 2015, 39(4): 782-788. DOI:10.7541/2015.102] |
| [4] |
Fujise D, Tsuji K, Fukushima N et al. Analytical aspects of cyanobacterial volatile organic compounds for investigation of their production behavior. Journal of Chromatography A, 2010, 1217(39): 6122-6125. DOI:10.1016/j.chroma.2010.07.008 |
| [5] |
Xu Q, Yang L, Yang W et al. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicology and Environmental Safety, 2017, 135: 191-200. DOI:10.1016/j.ecoenv.2016.09.027 |
| [6] |
Zuo Z, Zhu Y, Bai Y et al. Volatile communication between Chlamydomonas reinhardtii cells under salt stress. Biochemical Systematics and Ecology, 2012, 40: 19-24. DOI:10.1016/j.bse.2011.09.007 |
| [7] |
Zuo Z, Zhu Y, Bai Y et al. Acetic acid-induced programmed cell death and release of volatile organic compounds in Chlamydomonas reinhardtii. Plant Physiology and Biochemistry, 2012, 51: 175-184. DOI:10.1016/j.plaphy.2011.11.003 |
| [8] |
Fink P, Von Elert E, Jüttner F. Volatile foraging kairomones in the tittoral zone:attraction of an herbivorous freshwater gastropod to algal odors. Journal of Chemical Ecology, 2006, 32: 1867-1881. DOI:10.1007/s10886-006-9115-y |
| [9] |
Hasegawa M, Nishizawa A, Tsuji K et al. Volatile organic compounds derived from 2-keto-acid decarboxylase in Microcystis aeruginosa. Microbes Environments, 2012, 27: 525-528. DOI:10.1264/jsme2.ME12099 |
| [10] |
Conley DJ, Paer HW, Howarth RW et al. Controlling eutrophication:nitrogen and phosphorus. Science, 2009, 323: 1014-1015. DOI:10.1126/science.1167755 |
| [11] |
Qian ZL, Wang HJ, Sun JQ. The hindcast of winter and spring Arctic and Antarctic Oscillation with the coupled climate models. Acta Meteorologica Sinica, 2011, 25: 340-354. DOI:10.1007/s13351-011-0309-z |
| [12] |
Tekile A, Kim I, Kim J. Mini-review on river eutrophication and bottom improvement techniques, with special emphasis on the Nakdong River. Journal of Environmental Sciences-China, 2015, 30: 113-121. DOI:10.1016/j.jes.2014.10.014 |
| [13] |
Lin P, Klump JV, Guo L. Dynamics of dissolved and particulate phosphorus influenced by seasonal hypoxia in Green Bay, Lake Michigan. Science of the Total Environment, 2016, 541: 1070-1082. DOI:10.1016/j.scitotenv.2015.09.118 |
| [14] |
Steinhart GS, Likens GE, Soto D. Physiological indicators of nutrient deficiency in phytoplankton in southern Chilean lakes. Hydrobiologia, 2002, 489: 21-27. DOI:10.1023/A:1023271331086 |
| [15] |
Lu L, Zhao CX, Chen ZX et al. Analysis of nitrogen and phosphorus contents and assessmenton potential eutrophication of water in Xinkai Lake. Heilongjiang Science, 2011, 2(3): 1-3. [卢玲, 赵彩霞, 陈中祥等. 兴凯湖水域水体氮、磷含量特征及潜在性富营养化评价. 黑龙江科学, 2011, 2(3): 1-3.] |
| [16] |
Shen CN, Jiang ZJ, Cui Y et al. Analysis of nitrogen and phosphorus contents with assessment on potential eutrophication in the net-cage culture area in Tangdao Bay. Marine Fisheries Research, 2007, 28(3): 99-104. [沈春宁, 蒋增杰, 崔毅等. 唐岛湾网箱养殖区水体氮、磷含量特征及潜在性富营养化评价. 海洋水产研究, 2007, 28(3): 99-104.] |
| [17] |
Xu H, Wu YL, Yang GJ et al. Tolerance of Microcystis aeruginosa and Scendesmus obliquus to nitrogen and phosphorus deficiency. Ecological Science, 2014, 33(5): 879-884. [许海, 吴雅丽, 杨桂军等. 铜绿微囊藻、斜生栅藻对氮磷饥饿的耐受能力研究. 生态科学, 2014, 33(5): 879-884.] |
| [18] |
Lichtenthaler HK, Welburn A. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions, 1983, 603: 591-592. |
| [19] |
Zuo Z, Chen Z, Zhu Y et al. Effects of NaCl and Na2CO3 stresses on photosynthetic ability of Chlamydomonas reinhardtii. Biologia, 2014, 69(10): 1314-1322. |
| [20] |
Peers G, Truong TB, Ostendorf E et al. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature, 2009, 462: 518-522. DOI:10.1038/nature08587 |
| [21] |
Williamson GB, Richardson D. Bioassays for allelopathy:measuring treatment responses with independent controls. Journal of Chemical Ecology, 1988, 14: 181-187. DOI:10.1007/BF01022540 |
| [22] |
Bonsang B, Gros V, Peeken I et al. Isoprene emission from phytoplankton monocultures:the relationship with chlorophyll-a, cell volume and carbon content. Environmental Chemistry, 2010, 7: 554-563. DOI:10.1071/EN09156 |
| [23] |
Meskhidze N, Sabolis A, Reed R et al. Quantifying environmental stress-induced emissions of algal isoprene and monoterpenes using laboratory measurements. Biogeosciences, 2015, 12: 637-651. |
| [24] |
Dzialowski AR, Smith VH, Huggins DG et al. Development of predictive models for geosmin-related taste and odor in Kansas, USA, drinking water reservoirs. Water Research, 2009, 43: 2829-2840. DOI:10.1016/j.watres.2009.04.001 |
| [25] |
Holopainen JK. Can forest trees compensate for stress-generated growth losses by induced production of volatile compounds. Tree Physiology, 2011, 31(12): 1356-1377. DOI:10.1093/treephys/tpr111 |
| [26] |
Teuber M, Zimmer I, Kreuzwieser J et al. VOC emission of grey poplar leaves as affected by salt stress and different N sources. Plant Biology, 2008, 10(1): 86-96. DOI:10.1111/j.1438-8677.2007.00015.x |
| [27] |
Kivimäenpää M, Riikonen J, Ahonen V et al. Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environmental and Experimental Botany, 2013, 90: 32-42. DOI:10.1016/j.envexpbot.2012.11.004 |
| [28] |
Hartikainen K, Riikonen J, Nerg AM et al. Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environmental and Experimental Botany, 2012, 84: 33-43. DOI:10.1016/j.envexpbot.2012.04.014 |
| [29] |
Maffei ME. Sites of synthesis, biochemistry and functional role of plant volatiles. South African Journal of Botany, 2010, 76(4): 612-631. DOI:10.1016/j.sajb.2010.03.003 |
| [30] |
Schnitzler JP, Louis S, Behnke K et al. Poplar volatiles-biosynthesis, regulation and (eco) physiology of isoprene and stress-induced isoprenoids. Plant Biology, 2010, 12(2): 302-316. |
| [31] |
Morcuende R, Bari R, Gibon Y et al. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant, Cell and Environment, 2007, 30: 85-112. DOI:10.1111/pce.2007.30.issue-1 |
| [32] |
Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology, 2006, 57: 303-333. DOI:10.1146/annurev.arplant.57.032905.105228 |
| [33] |
Cheesbrough TM, Kolattukudy PE. Alkane biosynthesis by decarbonylation of aldehydes catalyzed by a particulate preparation from Pisum sativum. Proceedings of the National Academy of Sciences of the United States of America, 1984, 81: 6613-6617. DOI:10.1073/pnas.81.21.6613 |
| [34] |
Schirmer A, Rude MA, Li X et al. Microbial biosynthesis of alkanes. Science, 2010, 329: 559-562. DOI:10.1126/science.1187936 |
| [35] |
Zhang J, Lu X, Li JJ. Conversion of fatty aldehydes into alk(a/e)nes by in vitro reconstituted cyanobacterial aldehyde-deformylating oxygenase with the cognate electron transfer system. Biotechnology for Biofuels, 2013, 6: 86. DOI:10.1186/1754-6834-6-86 |
| [36] |
Klähn S, Baumgartner D, Pfreundt U et al. Alkane biosynthesis genes in cyanobacteria and their transcriptional organization. Frontiers in Bioengineering and Biotechnology, 2014, 2: 1-12. DOI:10.12970/2311-1755.2014.02.01.1 |
| [37] |
Baldwin IT, Halitschke R, Paschold A et al. Volatile signaling in plant-plant interactions:"talking trees" in the genomics era. Science, 2006, 311: 812-815. DOI:10.1126/science.1118446 |
| [38] |
Zuo Z, Zhang R, Gao P et al. Allelopathic effects of Artemisia frigida Willd. on growth of pasture grasses in Inner Mongolia, China. Biochemical Systematics and Ecology, 2011, 39: 377-383. DOI:10.1016/j.bse.2011.05.010 |
| [39] |
Zhang R, Zhang W, Zuo Z et al. Inhibition effects of volatile organic compounds from Artemisia frigida Willd. on the pasture grass intake by lambs. Small Ruminant Research, 2014, 121: 248-254. DOI:10.1016/j.smallrumres.2014.06.001 |
| [40] |
Ikawa M, Sasner JJ, Haney JF. Activity of cyanobacterial and algal odor compounds found in lake waters on green alga Chlorella pyrenoidosa growth. Hydrobiologia, 2001, 443: 19-22. DOI:10.1023/A:1017535801766 |
| [41] |
Xian QM, Chen HD, Zou HX et al. Allelopathic activity of volatile substance from submerged macrophytes on Microcystin aeruginosa. Acta Ecologica Sinica, 2006, 26(11): 3549-3554. [鲜啟鸣, 陈海东, 邹惠仙等. 沉水植物中挥发性物质对铜绿微囊藻的化感作用. 生态学报, 2006, 26(11): 3549-3554. DOI:10.3321/j.issn:1000-0933.2006.11.004] |
| [42] |
Ivanov AG, Hurry V, Sane PV et al. Reaction center quenching of excess light energy and photoprotection of photosystem Ⅱ. Journal of Plant Biology, 2008, 51: 85-96. DOI:10.1007/BF03030716 |
| [43] |
Zhang R, Zuo Z, Gao P et al. Allelopathic effects of VOCs from Artemisia frigida Willd. on regeneration of pasture grasses in Inner Mongolia, China. Journal of Arid Environments, 2012, 87: 212-218. DOI:10.1016/j.jaridenv.2012.04.008 |
| [44] |
Zhao J, Yang L, Zhou L et al. Inhibitory effects of eucalyptol and limonene on the photosynthetic abilities in Chlorella vulgaris (Chlorophyceae). Phycologia, 2016, 55: 696-702. DOI:10.2216/16-38.1 |
| [45] |
Zhou L, Zhao JX, Xu QH et al. Effects of eucalyptol and limonene on the photosynthetic abilities in Chlamydomonas reinhadtii. Acta Hydrobiologica Sinica, 2016, 40(6): 294-300. [周律, 赵静娴, 徐庆欢等. 桉树脑和柠檬烯对莱茵衣藻光合特性的影响. 水生生物学报, 2016, 40(6): 294-300.] |
| [46] |
Chang DW, Hsieh ML, Chen YM et al. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to β-cyclocitral. Journal of Hazardous Materials, 2011, 185: 1214-1220. DOI:10.1016/j.jhazmat.2010.10.033 |
| [47] |
Wang X, Kolattukudy PE. Solubilization and purification of aldehyde-generating fatty acyl-CoA reductase from green alga Botryococcus braunii. FEBS Letters, 1995, 370: 15-18. DOI:10.1016/0014-5793(95)00781-4 |
| [48] |
Walsh K, Dunstan RH, Murdoch RN et al. Bioaccumulation of pollutants and changes in population parameters in the gastropod Mollusc austrococh. Archives of Environmental Contamination and Toxicology, 1994, 26: 367-373. |
| [49] |
Gómez-Tenorio MA, Zanón MJ, de Cara M et al. Efficacy of dimethyl disulfide (DMDS) against Meloidogyne sp. and three formae speciales of Fusarium oxysporum under controlled conditions. Crop Protection, 2015, 78: 263-269. DOI:10.1016/j.cropro.2015.09.013 |
 2018, Vol. 30
2018, Vol. 30 

