(2: 中国科学院大学, 北京 100049)
(2: University of Chinese Academy of Sciences, Beijing 100049, P. R. China)
浮游动物是湖泊生态系统中的重要组成部分,其生长和繁殖受食物、捕食等生物因素和水温、光照等非生物因素的影响[1-3].食物的影响包括食物数量和质量,食物质量对浮游动物生长和繁殖影响的相关研究受到了广泛关注[4-6].食物质量指的是食物大小、元素比、生物化学组成和毒性有无等[7-9],其中食物碳磷比(C/P)与食物质量密切相关[10-11].湖泊中不同种类浮游动物的C/P不同且保持相对稳定,而其食物C/P变化很大[8, 12-14].不同种类的浮游动物选择食物的能力也不同,部分滤食性枝角类选择不同质量(如不同C/P)食物的能力较弱,因此对食物C/P要求高的种类对食物质量的变化更敏感[10, 15-17].溞(Daphnia spp.)是目前所测量的C/P最低的浮游动物[12],其对于食物C/P的变化很敏感[17-18],而象鼻溞(Bosmina spp.)对于食物C/P的变化不敏感[8].食物C/P过高( > 300)或者过低( < 40)都会对溞的生长和繁殖产生抑制作用[19-21].
同型溞(Daphnia similis)在我国淡水湖泊中广泛分布,是湖泊春季枝角类优势种之一[22],目前尚没有关于食物质量(如C/P)对其影响的报道.本研究通过用不同C/P的斜生栅藻(Scenedesmus obliquus)喂食同型溞,测定同型溞生长和繁殖的差异性,分析食物质量对同型溞生长和繁殖的影响,为探索自然条件下食物C/P大小对大型枝角类溞种群的影响提供依据.
1 材料和方法 1.1 斜生栅藻和同型溞的培养实验用斜生栅藻购置于中国科学院水生生物研究所藻种库,使用COMBO[23]培养基培养.培养条件为温度25℃,光照强度2000 lx,光照周期L: D=14 h: 10 h.在培养不同C/P斜生栅藻之前,取斜生栅藻母液以3000转/min的速度离心10 min,去掉上清液,加无菌水再次离心去掉上清液,随后加入到无氮无磷的COMBO培养基中,饥饿培养2 d.
基于COMBO培养基,将磷浓度分别设置为0、1、5和50 μmol/L.将经过饥饿培养的斜生栅藻接种至培养基中,接种密度为5×105 cells /ml.每2 d取藻液用分光光度计在685 nm处测定其吸光度以观察其密度相对变化.斜生栅藻密度达到对数期后结束培养,取藻液以3000转/min的速度离心10 min,去掉上清液,加无菌水再次离心去掉上清液,避光4℃保存.斜生栅藻的碳、磷含量测定均按照固体碳、磷测定方法进行,其中碳含量的测定采用元素分析仪(EA3000,意大利),磷含量的测定采用电感耦合等离子体发射光谱仪(Prodigy,美国).
同型溞采自太湖,于1 L烧杯中使用COMBO培养基并加入1 mg C/L斜生栅藻单克隆培养.培养条件为温度25℃,光照强度1200 lx,光照周期L: D=14 h: 10 h.在实验开始前一天将即将孵化幼体的同型溞移入到无氮无磷的COMBO培养基中,获取出生24 h以内的幼溞用于实验.
1.2 实验处理用4种不同C/P的斜生栅藻喂食同型溞,分别为C1、C2、C3和C4处理(表 1).每个处理设置4个平行,每个平行放入10只出生24 h以内的幼溞.在250 ml烧杯中培养,培养基体积为200 ml,食物浓度均控制在1 mg C/L.培养条件为温度25℃,光照强度1200 lx,光照周期L: D=14 h: 10 h.
| 表 1 不同磷浓度COMBO培养基培养的斜生栅藻碳、磷含量及其比例 Tab.1 The carbon(C) and phosphorus(P) contents and C/P ratios of Scenedesmus obliquus cultured by different P concentrations of COMBO medium |
每天用目镜带刻度的显微镜(Olympus X31,日本)测定同型溞的体长.每个烧杯挑取1只同型溞,每个处理的4只混在一起测定干重,每天更换培养基.干重的测定方法为用已称重的铝箔小杯盛装同型溞,在60℃下烘24 h,在十万分之一电子天平(CPA225D,德国)上称重.
培养第4 d,C3和C4处理组的同型溞开始怀卵,培养第9 d,C2处理组的同型溞开始怀卵,每天观察怀卵状况,记录所产幼仔只数并将其移除. C3和C4处理组与C2处理组分别于培养第9 d和第13 d获得第2成龄期产仔量后结束培养,测定同型溞的磷含量.培养第6~8 d不测同型溞的干重.
1.3 数据统计分析数据分析在SPSS 19.0软件中进行,不同处理同型溞体长和平均产仔量之间的差异使用单因素方差分析法(One-way ANOVA)进行分析.不同C/P斜生栅藻处理下同型溞的生长速率利用公式g=(ln mt-ln m0)/t计算[7],其中,g表示生长速率,mt表示培养t天的同型溞平均干重,m0表示初始的同型溞平均干重,t表示培养的天数.
2 实验结果 2.1 不同C/P的斜生栅藻对同型溞生长和磷含量的影响培养1 d后不同处理组同型溞的体长即显示出差异,C1与C2处理组差异不显著(P=0.94),C3与C4处理组差异不显著(P=0.86),但C1、C2处理组与C3、C4处理组差异均显著(P < 0.05),后期体长的差异显著性与第1 d相同.培养9 d后,高C/P斜生栅藻的C1和C2处理组同型溞的体长分别为1.48±0.07和1.70±0.12 mm,低C/P斜生栅藻的C3和C4处理组同型溞的体长分别为2.52±0.03和2.52±0.04 mm(图 1).
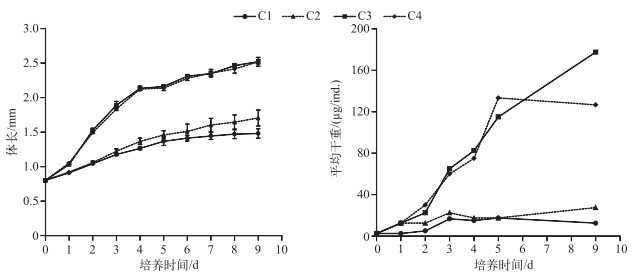
|
图 1 不同C/P斜生栅藻处理下同型溞的体长及平均干重随培养天数的变化 Fig.1 Changes in the body length and average dry weight of Daphnia similis cultured by different C/P ratios of Scenedesmus obliquus during the experiment |
C1、C2、C3和C4处理组培养9 d后同型溞的平均干重分别为12.5、27.5、177.5和127.0 μg/ind.. C1~C4处理组培养的同型溞在0~9 d内的平均生长速率分别为0.15、0.27、0.47和0.44 d-1.培养结束后C1~C4处理组同型溞的磷含量占干重的比例分别为0.35 %、0.79 %、1.43 %和1.11 % (图 2).

|
图 2 不同C/P斜生栅藻处理下同型溞的平均生长速率及磷含量 Fig.2 The average growth rate and body P content of Daphnia similis in four treatments with different C/P ratios of Scenedesmus obliquus |
C1处理组整个培养过程同型溞都没有产仔. C2处理组同型溞在培养第8 d首次怀卵,首次怀卵体长为1.64±0.11 mm,培养第9 d首次产仔. C3和C4处理组在培养第4 d首次怀卵,首次怀卵体长分别为2.13±0.02和2.13±0.04 mm(图 3),培养第5 d首次产仔. C2处理组与C3和C4处理组首次怀卵体长差异显著(P < 0.05),C3与C4处理组首次怀卵体长的差异不显著(P=0.99).
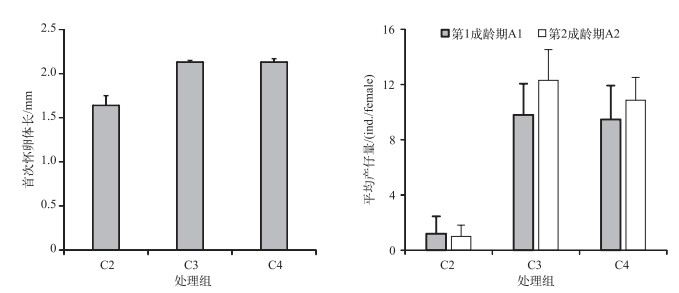
|
图 3 不同C/P斜生栅藻处理下同型溞首次怀卵体长和平均产仔量 Fig.3 The female body length at first pregnancy and average number of offspring of Daphnia similis in three treatments with different C/P ratios of Scenedesmus obliquus (A1: first adult instar; A2: second adult instar) |
C2处理组第1成龄期A1(培养第9~11 d)的平均产仔量为1.2±1.3 ind./female,C3和C4处理组A1期(培养第5~7 d)的平均产仔量分别为9.8±2.3和9.5±2.5 ind./female. C2处理组与C3和C4处理组A1期产仔量的差异显著(P < 0.05),C3与C4处理组差异不显著(P=0.98). C2处理组第2成龄期A2(培养第12~13 d)的平均产仔量为1.0±0.8 ind./female,C3和C4处理组A2期(培养第8~9 d)的平均产仔量分别为12.3±2.2和10.9±1.7 ind./female,差异显著性与A1期相同(图 3).
3 讨论 3.1 不同C/P的斜生栅藻对同型溞生长和磷含量的影响与低C/P(120)食物相比,高C/P( > 300)食物处理条件下溞的生长速率降低[7, 24].本研究中高C/P(512.3和881.8)处理条件下同型溞的生长速率低于低C/P(124.3)处理下的生长速率. DeMott等用不同C/P的栅藻(Scenedesmus acutus)喂食出生3 d的大型溞(Daphnia magna)3 d,食物C/P为900时的生长速率(0.17 d-1)低于食物C/P为164时的生长速率(0.50 d-1)[7]. C/P过高( > 300)的食物对溞生长产生抑制作用,其原因可能是食物C/P过高时溞吸收的磷不能满足其生理需求[7, 18],也可能是食物C/P过高时溞需要通过呼吸和排泄释放过多的碳[12, 25],或者是C/P过高的食物内部结构发生改变(如藻细胞壁变厚)导致可食性降低[15, 26].溞的C/P平均为93±20[27-29],食物C/P过低(低于溞的C/P)条件下溞的生长速率也会下降[21].本研究中斜生栅藻C/P为42.4处理组的同型溞生长速率低于C/P为124.3处理组的生长速率. Plath等用不同C/P的斜生栅藻喂食出生24 h以内的大型溞6 d,食物C/P为30时的生长速率(0.15 d-1)低于食物C/P为160时的生长速率(0.48 d-1)[17]. C/P过低( < 40)的食物对溞生长产生抑制作用,原因可能是食物C/P过低时溞摄食减慢导致碳含量不能满足其需求[17],也可能是食物C/P过低时溞需要排泄和解毒食物中过多的磷[21].因此,食物C/P过高( > 300)或者过低( < 40)都会抑制溞的生长[19, 30].
与低C/P(120)食物相比,食物C/P过高( > 300)条件下溞体的磷含量下降[24, 31].本研究中斜生栅藻C/P为512.3处理组的同型溞磷含量低于C/P为124.3处理组的磷含量. DeMott用不同C/P的镰形纤维藻(Ankistrodesmus falcatus)喂食出生24 h以内的大型溞5 d,食物C/P为1000时大型溞磷含量(0.84 %)低于食物C/P为70时的磷含量(1.48 %)[24].石琛等用不同C/P的扁藻(Platymonas subcordiformis)喂食出生24 h以内的安氏伪镖水蚤(Pseudodiaptomus annandalei),食物C/P为375时安氏伪镖水蚤无节幼体阶段的平均磷含量(0.11 %)低于食物C/P为78时的磷含量(0.31 %)[32].当食物C/P增加时,溞吸收的磷减少,溞的磷含量降低[7, 33].
3.2 不同C/P的斜生栅藻对同型溞繁殖的影响食物C/P的升高会使溞的生物量增长变缓,首次产仔时间增长,产仔量降低[8, 34].本研究中斜生栅藻C/P为881.8处理条件下的同型溞在培养的13 d内没有产仔,C/P为512.3处理的同型溞在培养第9 d产仔. Sterner等用C/P极高(2266)的栅藻(S. acutus)培养短钝溞(Daphnia obtusa),在培养的14 d内都没有产仔[18]. C/P为881.8处理的同型溞在培养期间没有产仔可能是由于其首次产仔时间超过了培养期,也可能是该处理的斜生栅藻的C/P过高导致同型溞不能正常蜕皮和繁殖[15].本研究中高C/P处理(512.3)条件下同型溞的A2期产仔量显著低于低C/P处理(124.3)下的A2期产仔量. Van Donk等用不同C/P的莱茵衣藻(Chlamydomonas reinhardtii)喂食蚤状溞(Daphnia pulex),食物C/P为845处理条件的蚤状溞的A2期产仔量(3.0±1.0 ind./female)低于C/P为214处理条件的A2期产仔量(8.3±2.0 ind./female)[26].溞应对C/P过高的食物时会降低繁殖率和种群增长率[35-36].本研究还发现斜生栅藻C/P为42.4处理条件下的同型溞产仔量低于C/P为124.3处理条件下的产仔量,表明斜生栅藻C/P过低也会对同型溞的繁殖产生抑制作用,其作用机理尚需进一步研究.
4 结论湖泊中食物C/P会对浮游动物的生长和繁殖产生较大影响.本研究通过探索不同C/P的斜生栅藻对同型溞生长和繁殖的影响,揭示了与斜生栅藻C/P为124.3处理条件相比,斜生栅藻C/P过高(512.3和881.8)及C/P过低(42.4)条件下,同型溞生长速率降低,磷含量减少,产仔量降低.这对研究食物质量高低对大型枝角类生长和繁殖的影响有一定的指导作用.针对不同C/P的食物对不同种浮游动物生长和繁殖的影响有待进一步研究.
致谢: 王文侠、薛庆举、王秀娟、苏小妹、蒋世雄在实验过程中给予了帮助,在此表示衷心感谢.| [1] |
DeMott WR, Gulati RD. Phosphorus limitation in Daphnia: Evidence from a long term study of three hypereutrophic Dutch lakes. Limnology and Oceanography, 1999, 44(6): 1557-1564. DOI:10.4319/lo.1999.44.6.1557 |
| [2] |
Persson J. Food quality effects on zooplankton growth and energy transfer in pelagic freshwater food webs. Uppsala: Uppsala University, 2007.
|
| [3] |
Hesson DO. Efficiency, energy and stoichiometry in pelagic food webs: Reciprocal roles of food quality and food quantity. Freshwater Reviews, 2008(1): 43-57. |
| [4] |
DeMott WR, Van Donk E. Strong interactions between stoichiometric constraints and algal defenses: Evidence from population dynamics of Daphnia and algae in phosphorus-limited microcosms. Oecologia, 2013, 171: 175-186. DOI:10.1007/s00442-012-2404-y |
| [5] |
Hessen DO, Elser JJ, Sterner RW et al. Ecological stoichiometry-an elementary approach using basic principles. Limnology and Oceanography, 2013, 58(6): 2219-2236. DOI:10.4319/lo.2013.58.6.2219 |
| [6] |
Wei Lijun, Tang Longsheng, Li Shengnan et al. Effects of a simulated increase in atmospheric CO2 concentration on cladoceran zooplankton collected from Lake Taihu. Acta Ecologica Sinica, 2016, 36(7): 1846-1853. [魏利军, 汤龙升, 李胜男等. CO2升高对枝角类群落结构影响的原位模拟. 生态学报, 2016, 36(7): 1846-1853.] |
| [7] |
DeMott WR, Gulati RD, Siewertsen K. Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnology and Oceanography, 1998, 43(6): 1147-1161. DOI:10.4319/lo.1998.43.6.1147 |
| [8] |
Schulz KL, Sterner RW. Phytoplankton phosphorus limitation and food quality for Bosmina. Limnology and Oceanography, 1999, 44(6): 1549-1556. DOI:10.4319/lo.1999.44.6.1549 |
| [9] |
Meng Meiru, Deng Daogui, Jiang Qiang et al. Effect of Chlorella Vulgaris cultured by different nitrogen concentrations on the growth and reproduction of Moina Irrasa. Journal of Huaibei Normal University: Natural Science, 2013, 34(3): 47-50. [孟美如, 邓道贵, 蒋强等. 不同氮浓度培养的小球藻对发头裸腹溞生长与生殖的影响. 淮北师范大学学报:自然科学版, 2013, 34(3): 47-50.] |
| [10] |
Sterner RW. Daphnia growth on varying quality of Scenedesmus: Mineral limitation of zooplankton. Ecology, 1993, 74(8): 2351-2360. DOI:10.2307/1939587 |
| [11] |
Laspoumaderes C, Modenutti B, Elser JJ et al. Does the stoichiometric carbon: phosphorus knife edge apply for predaceous copepods?. Oecologia, 2015, 178: 557-569. DOI:10.1007/s00442-014-3155-8 |
| [12] |
Sterner RW, Elser JJ, Hessen DO. Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry, 1992, 17: 49-67. |
| [13] |
Urabe J, Watanabe Y. Possibility of N or P limitation for planktonic cladocerans. Limnology and Oceanography, 1992, 37(2): 244-251. DOI:10.4319/lo.1992.37.2.0244 |
| [14] |
Elser JJ, Hassett RP. A stoichiometric analysis of the zooplankton-phytoplankton interaction in marine and freshwater ecosystems. Nature, 1994, 370: 211-213. DOI:10.1038/370211a0 |
| [15] |
VanDonk E, Hessen DO. Grazing resistance in nutrient-stressed phytoplankton. Oecologia, 1993, 93: 508-511. DOI:10.1007/BF00328958 |
| [16] |
Sterner RW, Hessen DO. Algal nutrient limitation and the nutrition of aquatic herbivores. Annual Review of Ecology and Systematics, 1994, 25(4): 1-29. |
| [17] |
Plath K, Boersma M. Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology, 2001, 82(5): 1260-1269. DOI:10.1890/0012-9658(2001)082[1260:MLOZSC]2.0.CO;2 |
| [18] |
Sterner RW, Hagemeier DD, Smith WL et al. Phytoplankton nutrient limitation and food quality for Daphnia. Limnology and Oceanography, 1993, 38(4): 857-871. DOI:10.4319/lo.1993.38.4.0857 |
| [19] |
Olsen Y, Jensen A, Reinertsen H et al. Dependence of the rate of release of phosphorus by zooplankton on the P :C ratio in the food supply, as calculated by a recycling model. Limnology and Oceanography, 1986, 31(1): 34-44. DOI:10.4319/lo.1986.31.1.0034 |
| [20] |
Urabe J, Clasen J, Sterner RW. Phosphorus limitation of Daphnia growth: Is it real?. Limnology and Oceanography, 1997, 42(6): 1436-1443. DOI:10.4319/lo.1997.42.6.1436 |
| [21] |
Elser JJ, Kyle M, Learned J et al. Life on the stoichiometric knife-edge: Effects of high and low food C :P ratio on growth, feeding, and respiration in three Daphnia. Inland Waters, 2016, 6: 136-146. DOI:10.5268/IW |
| [22] |
Li Jing, Chen Feizhou. Preliminary analysis on population decline of Daphnia in summer and autumn in Lake Taihu. J Lake Sci, 2010, 22(4): 552-556. [李静, 陈非洲. 太湖夏秋季大型枝角类(Daphnia)种群消失的初步分析. 湖泊科学, 2010, 22(4): 552-556. DOI:10.18307/2010.0411] |
| [23] |
Kilham SS, Kreeger DA, Lynn SG et al. COMBO: A defined fresh water culture medium for algae and zooplankton. Hydrobiologia, 1998, 377: 147-159. DOI:10.1023/A:1003231628456 |
| [24] |
DeMott WR. Implications of element deficits for zooplankton growth. Hydrobiologia, 2003, 491: 177-184. DOI:10.1023/A:1024408430472 |
| [25] |
Darchambeau F, Faerovig PJ, Hessen DO. How Daphnia copes with excess carbon in its food. Oecologia, 2003, 136: 336-346. DOI:10.1007/s00442-003-1283-7 |
| [26] |
Van Donk E, Hessen DO. Altered cell wall morphology in nutrient-deficient phytoplankton and its impact on grazers. Limnology and Oceanography, 1997, 42(2): 357-364. DOI:10.4319/lo.1997.42.2.0357 |
| [27] |
Elser JJ, Urabe J. The stoichiometry of consumer-driven nutrient recycling: Theory, observations, and consequences. Ecology, 1999, 80(3): 735-751. DOI:10.1890/0012-9658(1999)080[0735:TSOCDN]2.0.CO;2 |
| [28] |
Elser JJ, Fagan WF, Denno RF et al. Nutritional constraints in terrestrial and freshwater food webs. Nature, 2000, 408: 578-580. DOI:10.1038/35046058 |
| [29] |
Brett MT, Muller-Navarra DC, Park SK. Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton. Limnology and Oceanography, 2000, 45(7): 1564-1575. DOI:10.4319/lo.2000.45.7.1564 |
| [30] |
Boersma M, Elser JJ. Too much of a good thing: On stoichiometrically balanced diets and maximal growth. Ecology, 2006, 87(5): 1325-1330. DOI:10.1890/0012-9658(2006)87[1325:TMOAGT]2.0.CO;2 |
| [31] |
Lehman JT, Naumoski T. Content and turnover rates of phosphorus in Daphnia pulex: Effect of food quality. Hydrobiologia, 1985, 128: 119-125. DOI:10.1007/BF00008731 |
| [32] |
Shi Chen, Lü Songhui, He Xuejia. Effect of phosphorus condition on growth and ingestion in Pseudodiaptomus annandalei. Acta Hydrobiologica Sinica, 2011, 35(3): 460-466. [石琛, 吕颂辉, 何学佳. 不同磷条件对安氏伪镖水蚤的生长及摄食的影响. 水生生物学报, 2011, 35(3): 460-466.] |
| [33] |
He XJ, Wang WX. Kinetics of phosphorus in Daphnia at different food concentrations and carbon: phosphorus ratios. Limnology and Oceanography, 2007, 52(1): 395-406. DOI:10.4319/lo.2007.52.1.0395 |
| [34] |
Acharya K, Kyle M, Elser J. Effects of stoichiometric dietary mixing on Daphnia growth and reproduction. Oecologia, 2004, 138(3): 333-340. DOI:10.1007/s00442-003-1444-8 |
| [35] |
Kilham SS, Kreeger DA, Goulden CE et al. Effects of algal food quality on fecundity and population growth rates of Daphnia. Freshwater Biology, 1997, 38: 639-647. DOI:10.1046/j.1365-2427.1997.00232.x |
| [36] |
Frost PC, Ebert D, Larson JH et al. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia, 2010, 162: 865-872. DOI:10.1007/s00442-009-1517-4 |
 2017, Vol. 29
2017, Vol. 29 

